• Strength of an Acid or Base
Strength of acids and bases depends on the no. of H+ions and OH–ions produced respectively.
With the help of a universal indicator we can find the strength of an acid or base as it shows different colours at different concentrations of hydrogen ions in a solution.
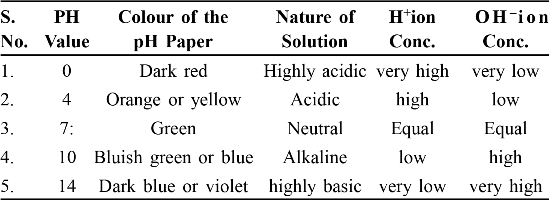
A scale for measuring hydrogen ion conc. in a solution is called pH scale has been developed.
pH = Potenz in German means power.
This scale measures from 0 (very acidic) to 14 (very alkaline) 7 Neutral (water in neutral).
pH paper : Is a paper which is used for measuring pH.
Variation of PH
– strong Acids give rise to more H+ions.
eg. HCl, H2SO4 and HNO3.
– Weak Acids give rise to less H+ ions
eg. CH3 COOH, H2 CO3 (Carbonic acid)
– Strong Bases – Strong bases give rise to more OH– ions.
eg. NaOH, KOH, Ca(OH)2
– Weak Bases : give rise to less OH– ions.
eg. NH4OH
• More about Salts
Salts and their derivation
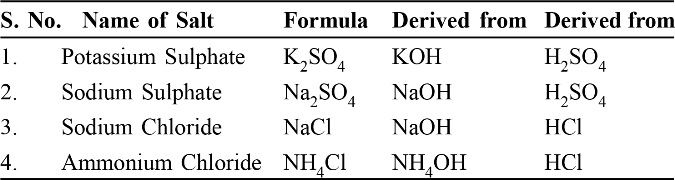
Note : NaCl and Na2 SO4 belong to the family of sodium salts as they have the same radicals. Similarly NaCl and KCl belong to the family of chloride salts.
Importance of pH in our daily life
• Importance of pH in our digestive system – Our stomach produces hydrochloric acid. This dilute hydrochloric acid help in digestion of good. In case of indigestion our stomach produces acid in a very large quantity because of which we feel pain and irritation in our stomach. To get relief from this pain antacids are used. These antacids neutralise the excess acid and we get relief.
• pH of Acid Rain : When pH of rain water is less than 5.6 it is called acid rain. When this acidic rain flows into rivers these were also get acidic, which causes a threat to the survival of aquatic life.
• pH of Soil : Plants require a specific range of pH for their healthy growth. If pH of soil of any particular place is less or more then normal than the farmers add suitable chemicals to it.
• Our body functions between the range of 7.0 to 7.8 living organisms can survive only in the narrow range of pH change.
• Tooth decay and pH : Bacteria present in the mouth produces acids by degradation of sugar and food particles remaining in the mouth. Using toothpaste which is generally basic, can neutralise the excess acid and prevent tooth decay.
• Bee sting or Nettle sting contains methanoic acid which causes pain and irritation. When we use a weak base like baking soda on it, we get relief.

• Chemicals from Common Salt
– Sodium chloride is called as common salt is used in our food. It is derived from seawater.
– Rock Salt is the brown coloured large crystals. This s mined like coal.
– Common salt is an important raw material for many materials of daily use such as.
Sodium hydroxide
Washing Soda
Bleaching Power.
• Sodium Hydroxide : NaOH, Common Name – caustic soda.
Latest Govt Job & Exam Updates: