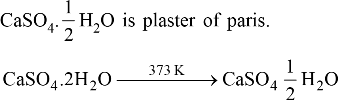
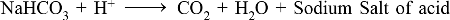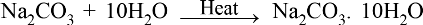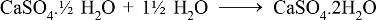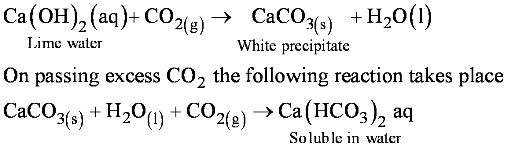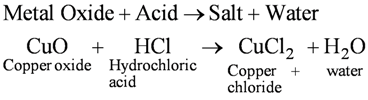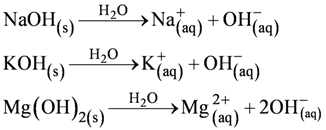• Action of Acids with metal Carbonates and metal bicarbonates
Metal Carbonate + Acid → Salt + Carbondioxide + Water
Na2CO3(S) + 2HCl(aq) → 2NaCl(aq) + H2O(l) + CO2(g)
Metal bicarbonate + Acid → Salt + Carbondioxide + Water
NaHCO3 + HCl → NaCl + CO2 + H2O
• Lime water Test : On passing the evolved CO2 gas is Passed through lime water,
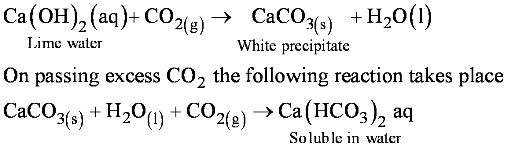
• Reaction of acids and bases with each other.
• Neutralisation Reactions
Base + Acid → Salt + Water
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
Neutralisation reaction : The reaction between an acid and a base to give salt and waste is called as neutralization reaction takes place when the effect of a base is nullified by an acid and vice versa to give salt and water.
• Reactions of metal oxides with acids
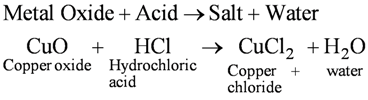
Note : Appearance of blue green colour of the solution because of formation of CuCl2.
Metallic oxides are said to be basic oxides because they give salt and water on reacting with acids.
• Reaction of Non Metallic Oxide with Base
Non metallic oxide + Base → Salt + Water
Ca(OH)2 + CO2 → CaCO3 + H2O
Note : Non Metallic oxides are said to be acidic in nature because on reacting with a base they produce salt and water.
• All acidic solutions conduct electricity because of formation of (H+ions in eq, solution.)
Refer activity 2.3 on page 22 of NCERT Book
– Glowing of bulb indicates that there is a flow of electric current through the solution.
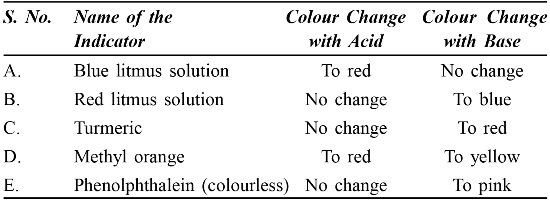
• Acids or bases in a Water Solution
Acids produce H+ ions in the presence of water
HCl + H2O → H3O+ + Cl–
H3O+ – Hydronium ion.
– H+ion cannot exist alone. It exists as H+(aq) or (H3O+) hydronium ion.
H+ + H2O → H3O+
– Bases provide (OH–) ions in the presence of water
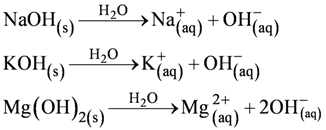
• Alkalis
All bases donot dissolve in water. An alkali is a base that dissolves in water. Common alkalis are
NaOH Sodium hydroxide
KOH Potassium hydroxide
Ca(OH)2 Calcium hydroxide
NH4OH Ammonium hydroxide
Note : All alkalis are bases but all bases are not alkalis.
• Precaution must be taken while mixing acid or base with water. The acid must always be added to water with constant stirring as it is a highly exothermic reaction.
When an acid or a base is mixed with water they become dilute. This results in the decrease in the concentration of H3O+ or OH– per unit volume in acids and bases respectively.