AIIMS Solved Paper-2019
Part A: Physics
1. A gun applied a force F on a bullet which is given by F = (100 – 0.5 × 105 t) N. The bullet emerges out with speed 400 m/s. Then find out the impulse exerted till force on the bullet becomes zero.
(a) 0.2 Ns
(b) 0.3 Ns
(c) 0.1 Ns
(d) 0.4 Ns
2. A transformer with turns ratio ![]() is connected to a 120 V AC supply. If p rimary and secondary circuit resistances are 15 kΩ and 1Ω respectively, then find out power of output.
is connected to a 120 V AC supply. If p rimary and secondary circuit resistances are 15 kΩ and 1Ω respectively, then find out power of output.
(a) 5.76 W
(b) 11.4 W
(c) 2.89 W
(d) 7.56 W
3. A proton is projected with velocity ![]() in a region where magnetic field
in a region where magnetic field ![]() and electric field
and electric field ![]() Then find out the net acceleration of proton
Then find out the net acceleration of proton
(a) 1400 m/s2
(b) 700 m/s2
(c) 1000 m/s2
(d) 800 m/s2
4. The given transistor operates in saturation region then what should be the value of VBB?
(Rout = 200 Ω, Rin = 100 kΩ, VCC = 3 V, VBE = 0.7 V, VCE = 0, β = 200)
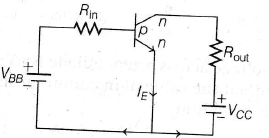
(a) 4.1 V
(b) 7.5 V
(c) 8.2 V
(d) 6.8 V
5. In figure, two parallel infinitely long current carrying wires are shown. If resultant magnetic field at point A is zero. Then determine the value of current I.

(a) 50 A
(b) 15 A
(c) 30 A
(d) 25 A
6. The temperature of food material in refrigerator is 4°C and temperature of environment is 15° If carnot cycle is used in its working gas, then find its carnot efficiency.
(a) 0.038
(b) 0.028
(c) 0.053
(d) 0.072
7. A string wave equation is given
y = 0.002 sin(300t – 15x) and mass density is (μ = 0.1 kg/m). Then find the tension force in the string.
(a) 30 N
(b) 20 N
(c) 40 N
(d) 45 N
8. If maximum energy is stored in a capacitor at t = 0, then find the time after which current in the circuit will be maximum.
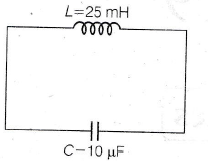
(a) π/2 ms
(b) π/4 ms
(c) π ms
(d) 2 ms
9. If voltage across a zener diode is 6V, then find out the value of maximum resistance in this condition.

(a) 2 kΩ
(b) 3 kΩ
(c) 5 kΩ
(d) 4 kΩ
10. Two circular loops having same radius (R = 10 cm) and same current ![]() are placed along same axis as shown. If the distance between their centres is 10 cm, find net magnetic field at point P.
are placed along same axis as shown. If the distance between their centres is 10 cm, find net magnetic field at point P.
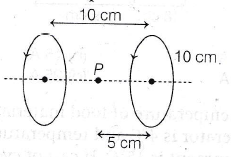
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
11. Initially spring in its natural length now a block at mass 0.25 kg is released then find out maximum force by system on the floor.

(a) 15 N
(b) 20 N
(c) 25 N
(d) 30 N
12. If 7 gm N2 is mixed with 20 gm Ar, there Cp/Cv of mixture will be
(a) 17/6
(b) 11/7
(c) 17/11
(d) 17/13
13. Distance of 5th dark fringe from centre is 4 mm. If D = 2 m, λ = 600 nm, then distance between slits is
(a) 1.35 mm
(b) 2.00 mm
(c) 3.25 mm
(d) 10.35 mm
14. A sphere pure rolls on a rough a inclined plane with initial velocity 2.8 m/s. Find the maximum distance on the inclined plane.
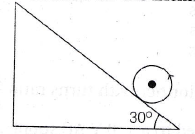
(a) 2.74 m
(b) 5.48 m
(c) 1.38 m
(d) 3.2 m
15. Find gravitational field at a distance of 2000 km from the centre of earth. (Given Rearth = 6400 km, r = 2000 km, Mearth = 6 × 1024 kg)
(a) 1.53 m/s2
(b) 7.12 m/s2
(c) 3.06 m/s2
(d) 1.8 m/s2
16. Two sources of sound S1 and S2 are moving towards and away from a stationary observer with the same speed respectively. Observer detects 3 beats per second. Find speed of sources (approximately.)

Given, f1 = f2 = 500 Hz, speed of sound in air = 300 m/s
(a) 1 m/s
(b) 2 m/s
(c) 3 m/s
(d) 4 m/s
17. 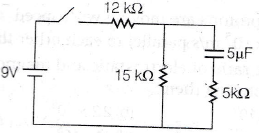
In the given circuit, find charge of capacitor after 1s of opening the switch at t = ∞.
(a) 20 e−10 μC
(b) 25 e−10 μC
(c) 30 e−10 μC
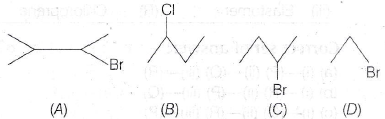
(d) 35 e−10 μC
18. In an isobaric process, the work done by a di-atomic gas is 10 J, the heat given to the gas will be
(a) 35 J
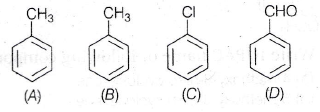
(b) 30 J
(c) 45 J
(d) 60 J
19. A capacitor of capacitance 15 μF having dielectric slab of εr = 2.5, dielectric strength 30 MV/m and potential difference = 30 V. Calculate the area of the plate.
(a) 6.7 × 10−4 m2
(b) 4.2 × 10−4 m2
(c) 8.0 × 10−4 m2
(d) 9.85 × 10−4 m2
20. For a wire ![]() and length of wire is ℓ = 5 cm. If potential difference of 1 V is applied across it, then current through wire will be
and length of wire is ℓ = 5 cm. If potential difference of 1 V is applied across it, then current through wire will be
(R = Resistance)
(a) 40 A
(b) 4 A
(c) 25 A
(d) 2.5 A
21. If modulation index, μ = 1/2 and Vm = 2, then VC is
(a) 4
(b) 2
(c) 6
(d) 8
22. A body of mass 5 × 103 kg moving with speed 2 m/s collides with a body of mass 15 × 103 kg inelastically and sticks to it. Then loss in KE of the system will be
(a) 7.5 kJ
(b) 15 kJ
(c) 10 kJ
(d) 5 kJ
23. If local length of objective and eye lenses are 10 cm and 10 mm respectively and tube length is 11 cm then angular magnification of telescope is
(a) 10
(b) 5
(c) 100
(d) 50
24. An electron is moving in a circle of radius 2m with speed of 4 m/s. Find the acceleration of the electron.
(a) 8 m/s2
(b) 4 m/s2
(c) 16 m/s2
(d) 10 m/s2
25. If a small orifice is made at a height of 0.25 m from the ground, the horizontal range of water stream will be
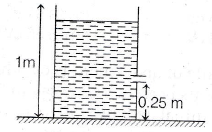
(a) 46.5 cm
(b) 56.6 cm
(c) 76.6 cm
(d) 86.6 cm
26. Calculate the mean percentage error in five observations,
80.0, 80.5, 81.0, 81.5, 82
(a) 0.74%
(b) 1.74%
(c) 0.38%
(d) 1.38%
27. Calculate the focal length of given lens if the magnification is −5.

(a) 6.66 cm
(b) 5.44 cm
(c) 3.88 cm
(d) 1.38 cm
28. In a LCR series circuit source voltage is 120 V and voltage in inductor 50 V and resistance is 40 V, then determine voltage in the capacitor
(a) VC = 10(5 – 8√2)
(b) VC = 10(5 + 8√2)
(c) VC = 20(5 + 8√2)
(d) VC = 10(5 + 7√2)
29. Determine the pressure difference in tube of non-uniform cross sectional area as shown in figure.
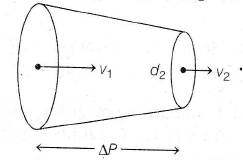
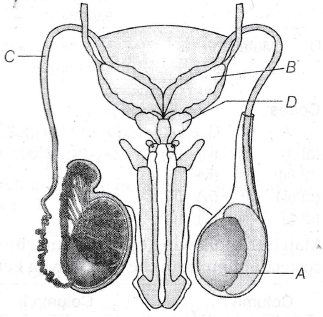
∆P = ?, d1 = 5 cm, v1 = 4 m/s, d2 = 2 cm, v2 = ?
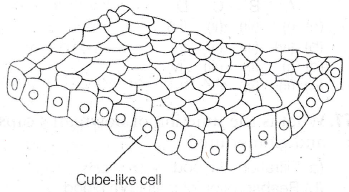
(a) 304200 Pa
(b) 304500 Pa
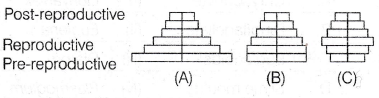
(c) 302500 Pa
(d) 303500 Pa
30. α-particle is revolving in a circular path with radius r with speed v, then find the value of magnetic dipole moment.
(a) 2 evr
(b) evr
(c) 3 evr
(d) 4 evr
31. 15 eV is given to electron in 4th orbit then find its final energy when it comes out of H-atom.
(a) 14.15 eV
(b) 13.6 eV
(c) 12.08 eV
(d) 15.85 eV
32. If half life of an element is 69.3 h, then how much of its percent will decay in 10th to 11th h. Initial activity = 50 μ Ci
(a) 1%
(b) 2%
(c) 3%
(d) 4%
33. If the speed of sound in air is 330 m/s then, find the number of tones present in an open organ pipe of length 1 m whose frequency is ≤ 1000 Hz.
(a) 2
(b) 4
(c) 8
(d) 6
34. A light of wavelength 500 nm is incident on a Young’s double slit. The distance between slit and screen is D = 1.8 m and distance between slits is d = 0.4 mm. If screen moves with a speed of 4 m/s, then with what speed first maxima will move?
(a) 5 mm/s
(b) 4 mm/s
(c) 3 mm/s
(d) 2 mm/s
35. In an isothermal process 2 water drops of radius 1 mm are combined to form a bigger drop. Find the energy change in this process if T = 0.1 N/m.
(a) 1 μJ
(b) 0.5 μJ
(c) 0.25 μJ
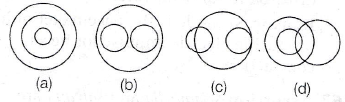
(d) 0.75 μJ
36. If temperature of Sun = 6000 K, radius of Sun is 7.2 × 105 km, radius of Earth = 6000 km and distance between Earth and Sun = 15 × 107 Find intensity of light on Earth.
(a) 19.2 × 1016
(b) 12.2× 1016
(c) 18.3× 1016
(d) 92× 1016
37. Calculate radiation power for sphere whose temperature is 227°C, radius 2 m and emissivity 0.8.
(a) 142.5 kW
(b) 1500 W
(c) 1255 W
(d) 1575 W
38. If two protons are moving with speed v = 4.5 × 105 m/s parallel to each other then find the ratio of electrostatic and magnetic force between them
(a) 4.4 × 105
(b) 2.2× 105
(c) 3.3× 105
(d) 1.1× 105
39. An ideal gas initially at pressure 1 bar is being compressed from 30 m3 to 10 m3 volume and its temperature decreases from 320 K to 280 K, then find final pressure of the gas.
(a) 2.625 bar
(b) 3.4 bar
(c) 1.325 bar
(d) 4.5 bar
40. A current of 10 A is passing through a metallic wire of cross-sectional area 4 × 10−6 m2. If the density of the aluminium conductor is 2.7 gm/cc considering aluminium gives 1 electron per atom for conduction, then find the drift velocity of the electrons if molecular weight of aluminium is 27 gm.
(a) 1.6 × 10−4 m/s
(b) 3.6 × 10−4 m/s
(c) 2.6 × 10−4 m/s
(d) 1.5× 10−4 m/s
Direction (Q. Nos. 41-60) In each of the following questions a statement of assertion is given followed by the corresponding statement of reason. Of the statements, mark the correct answer as
(a) If both assertion and reason are true and reason is the correct explanation of assertion
(b) If both assertion and reason are true, but reason is not the correct explanation of assertion.
(c) If assertion is true, but reason is false.
(d) If both assertion and reason are false.
41. Assertion A metallic surface is moved in and out in magnetic field them emf is induced in it.
Reason Eddy current will be produced in a metallic surface moving in and out of magnetic field.
42. Assertion There is no loss in energy in elastic collision.
Reason Linear momentum is conserved in elastic collision.
43. Assertion Paramagnetic substances get poorly attracted in magnetic field.
Reason Because magnetic dipoles are aligned along external magnetic field weakly.
44. Assertion Distance between position of bright and dark fringe remain same in YDSE.
Reason Fringe width, β = λD/d
45. Assertion In both radioactivity and photoelectric effect electrons may be ejected.
Reason In photoelectric effect and radioactivity emission occurs only of unstable elements.
46. Assertion Electron moving perpendicular to B will perform circular motion.
Reason Force by magnetic field is perpendicular to velocity.
47. Assertion In ionospheric reflection, phase change does not occur with the radio wave.
Reason The ionosphere reflection is similar to the total internal reflection in miraj.
48. Assertion Vibrational degree of freedom of a di-atomic gas molecule appears at every high temperature.
Reason Di-atomic gas two vibrational degree of freedom in one direction.
49. Assertion NH3 is liquidities more easily than CO2.
Reason Critical temperature of NH3 is more than CO2
50. Assertion In adiabatic process work is independent of the path.
Reason In adiabatic process work done is equal to negative of change in internal energy.
51. Assertion Water drops take spherical shape when falling freely.
Reason Water has minimum surface tension among all liquids.
52. Assertion Photodiode and solar cell work on same mechanism.
Reason Area is large for solar cell.
53. Assertion Sometimes insects can walk on water.
Reason The gravitational force on insect is balanced by force due surface tension.
54. Assertion Heavy water is used to slow neutron in nuclear reactor.
Reason It does not react with slow neutron and mass of deuterium is comparable to the neutron.
55. Assertion Incoming light reflected by earth is partially polarized.
Reason Atmospheric particle polarize the light.
56. Assertion Amplitude modulation shows more interference than frequency modulation with noise.
Reason Interference is function of amplitude of modulation wave with carrier-wave.
57. Assertion A charge particle is released from rest in magnetic field then it will move in a circular path.
Reason Work done by magnetic field is non zero.
58. Assertion A glass ball is dropped on concrete floor can easily get broken compared if its is dropped on wooden floor.
Reason On concrete floor glass ball will take less time to come to rest.
59. Assertion Even though net external force on a body is zero, momentum need not to converved.
Reason The internal interaction between particles of a body cancels out momentum of each other.
60. Assertion For an element generally N ≥ Z (N = number of neutrons, Z = atomic number)
Reason Neutrons always experience attractive nuclear force.
Part B : Chemistry
61. Out of BeF2, MgF2, CaF2, SrF2 which has maximum solubility?
(a) BeF2
(b) MgF2
(c) CaF2
(d) SrF2
62. Which of the following statement is correct for oleum?
(a) It is prepared by adsorption of SO3 in conc. H2SO4
(b) It contains O−O groups
(c) It has six OH groups
(d) None of the above
63. Which of the following can react with K2Cr2O7?
(a) SO3−2
(b) CO3−2
(c) SO4−2
(d) NO3−
64. Which element can have oxidation state from 4 to 6?
(a) Fe
(b) Mg
(c) Co
(d) Cr
65. Order of acidic nature
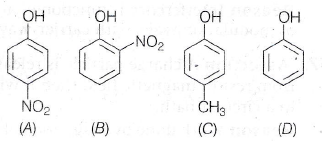
(a) A > C > D > B
(b) A > B > D > C
(c) A > B > C > D
(d) D > C > B > A
66. The vapour pressure of pure CHCl3 and CH2Cl2 are 200 and 41.5 atm respectively. The weight of CHCl3 and CH2Cl2 are respectively 11.9 g and 17 g. The vapour pressure of solution will be
(a) 80.5
(b) 79.5
(c) 94.3
(d) 105.5
67. Correct order of bond dissociation energy
(a) Br2 > Cl2
(b) F2 > Cl2
(c) I2 > F2
(d) F2 > I2
68. The tetrahedral voids are present in 0.5 mole of hcp crystal structure
(a) 3.6 × 1023
(b) 9 × 1023
(c) 3.6 × 1024
(d) 6.02 × 1023
69. Find out time period of 1st order reaction. When reaction complete 2/3rd. If the value of rate constant is 4.3 × 10−4
(a) 0.0025 × 103 sec
(b) 0.25 × 103 sec
(c) 0.025 × 103 sec
(d) 2.5 × 103 sec
70. Compare stability of free radicals
(I) CH3−CH−CH3
(II) ![]()
(III) CH2 – CH(CH3)2
(IV) CH2 – CH3
(a) II > I > III > IV
(b) II > I > IV > III
(c) I > II > III > IV
(d) IV > III > I > II
71. 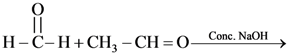 Find out the products of reaction
Find out the products of reaction
(a) CH3CO2Na and CH3OH
(b) CH3CH2OH + CH3OH
(c) CH3CH2OH and HCO2Na
(d) CH3CO2Na + HCO2Na
72. If boiling point of water is 100° How much gram of NaCl is added in 500 g of water to increase its boiling point of water by approx ![]()
(a) 2.812 g
(b) 28.12 g
(c) 14.06 g
(d) 7.03 g
73. (i) F3C – COHH, (ii) CH3COOH,
(iii) C6H5COOH, (iv) CH3CH2COOH
Correct order of pKa value is
(a) (i) > (iii) > (ii) > (iv)
(b) (iv) > (ii) > (iii) > (i)
(c) (iv) > (iii) > (ii) > (i)
(d) (i) > (ii) > (iv) > (iii)
74. The correct relation is
(a) ∆G = −RT ln K/Q
(b) ∆G = +RT ln K
(c) ∆G = −RT ln Q/K
(d) ∆G = +RT ln Q
75. [Co(C2O4)3]3− is a
(a) low spin complex
(b) paramagnetic
(c) high spin
(d) sp3d2-hyridized
76. Find empirical formula of the compound if M = 68% (atomic mass = 34) and remaining 32% oxygen.
(a) MO
(b) M2O
(c) MO2
(d) M2O3
77. A bulb is emitted electromagnetic radiation of 660 nm wavelength. The total energy of radiation is 3 × 10−18 The number of emitted photon will be
(h = 6.6 × 10−34) J × s, c = 3 × 108 m/s
(a) 1
(b) 10
(c) 100
(d) 1000
78. Yellow colour of chlorine water fades because of
(a) form HCl and HOCl
(b) chlorine gas escapes
(c) ClO2 + H2
(d) Cl2O
79. Which have melting point below 500°C?
(a) Ag, Cu
(b) Zn, Cd
(c) Cd, Cu
(d) Ag, Zn
80. Which of the following inert gas participate in chemical reaction?
(a) Xe
(b) He
(c) Ne
(d) None of these
81. Which of the following is correct order of packing efficiency?
(a) hcp = fcc > bcc > sc
(b) sc > bcc > hcp = fcc
(c) bcc > sc > hcp < fcc
(d) fcc = hcp > sc > bcc
82. Cell notation, 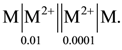 If value of E°cell is 4 volt
If value of E°cell is 4 volt ![]()
(a) 3.94 V
(b) 4.06 V
(c) 2.03 V
(d) 8.18 V
83. Which of the following has maximum iron content?
(a) Cast Iron
(b) Wrought Iron
(c) Pig Iron
(d) Stainless steel
84. pH of a salt solution of weak acid (pKa = 4) and weak base (pKb = 5) at 25° is
(a) 6.5
(b) 6
(c) 7
(d) 7.5
85. Correct increasing order for the wavelength of absorption in the visible region for the complexes of Co3+ is
(a) [Co(CN)6]3−, [Co(NH3)6] 3+, [Co(NH3)5 (H2O)]3+, [Co(NH3)5Cl]+2
(b) [Co(CN)6] 3−, [Co(NH3)6]3+, [Co(NH3)5Cl]2+, [Co(NH3)6]3+
(c) [Co(NH3)6]3+, [Co(CN)6] 3−, [Co(NH3)5(H2O)]3+, [Co(NH3)Cl]+2
(d) [Co(NH3)5Cl]+2, [Co(NH3)5(H2O)]3+, [Co(NH3)6]3+, [Co(CN)6] 3−
86. At 25°C, 1 mole of butane is heated then CO2 and H2O liquid is formed work done is
(a) 75.6 atm
(b) 85.6 atm
(c) 50.3 atm
(d) None of these
87. What is the activation energy (kJ/mol) for a reaction if its rate constant doubles when the temperature is raised from 300 K to 400 K of these (R = 8.314 Jmol−1K−1)
(a) 68.8
(b) 6.88
(c) 34.4
(d) 3.44
88. H2O2 is obtained by which of the following?
(a) BaO2
(b) MnO2
(c) SeO2
(d) TeO2
89. Which has least covalent radius?
(a) Mn
(b) Cu
(c) Zn
(d) Ni
90. Which give coloured carbonate precipitate?
(a) Hg22+
(b) Sr+2
(c) Bi+3
(d) Li+
91. Which of the following, number of lone pair at central atom zero XeO3, XeO2F2, XeO4, XeO3F2, XeF6?
(a) 2
(b) 3
(c) 4
(d) Zero
92. 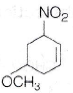
Write IUPAC name of following compound.
(a) 4-methoxy-6-nitro cyclohexene
(b) 5-methoxy-3-nitro cyclohexene
(c) 3-nitro-1-methoxy cyclohex-4-ene
(d) 3-nitro-5-methoxy cyclohexene
93. 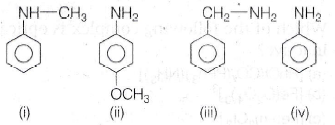
Correct order of basic strength is
(a) iii > ii > iv > i
(b) iv > iii > ii > i
(c) iii > ii > i > iv
(d) iii > i > ii > iv
94. Which is least soluble?
(a) Na2S
(b) MgS
(c) MgCl2
(d) NaCl
95. Correct order for reaction with alcoholic KOH
(a) A > B > C > D
(b) A > C > B > D
(c) D > B > C > A
(d) A > D > B > C
96. Which is most stable conformer of eathan-1, 2-diol?
(a) 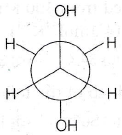
(b) 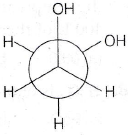
(c) 
(d) 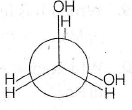
97. Which pair of elements has maximum electronegativity difference?
(a) Li and F
(b) Na and F
(c) Na and Br
(d) Na and Cl
98. Correct order of electrophillic substitution reaction is
(a) A > B > C > D
(b) D > B > A > C
(c) B > A > C > D
(d) B > A > D > C
99. Which of the following complex is optically inactive?
(a) [RhCl(CO)(PPh3)(NH3)]
(b) [Fe(C2O4)3]3−
(c) [Fe(en)2Cl2]
(d) [Pd(en)2Cl2]
100. Match the following columns.
Polymer Monomer
(i) Buna –S (P) Styrene
(ii) Ethylene glycol (Q) Terylene
(iii) Elastomer (R) Chloroprene
(a) (i) – (P) (ii) – (Q), (iii) – (R)
(b) (i) – (R) (ii) – (P), (iii) – (Q)
(c) (i) – (Q) (ii) – (R), (iii) – (P)
(d) (i) – (P) (ii) – (R), (iii) – (Q)
Direction (Q. Nos. 101-120) In each of the following questions a statement of assertion is given followed by the corresponding statement of reason. Of the statements, mark the correct answer as
(a) If both assertion and reason are true and reason is the correct explanation of assertion
(b) If both assertion and reason are true, but reason is not the correct explanation of assertion
(c) If assertion is true, but reason is false
(d) If both assertion and reason are false
101. Assertion The chemical properties of different isotope are same.
Reason Isotopes having same number of neutron.
102. Assertion Out of CrO3 and Al2O3, CrO3 having lower melting point than Al2O3.
Reason Oxidation state of Cr in CrO3 is high.
103. Assertion U is state function.
Reason T is an intensive property.
104. Assertion BO3−3 and SO3−2 are not isostructural.
Reason In SO32− sulphur has one lone pair of electron.
105. Assertion Tert-butyl methyl ether react with HBr to form tert butyl, (CH3)3 C−Br and CH3−OH, methanal.
Reason It follow SN1 mechanism.
106. Assertion a spherical water drops become flaton flatter surface.
Reason It become flat due to gravity.
107. Assertion Two sugar units joined by 1, 2-glycosidic bond in sucrose.
Reason It contains C1-glucose and C2-fructose glycosidic bond.
108. Assertion S2O72− and Cr2O72− both exist.
Reason Both have same valence electrons.
109. Assertion ZnO becomes yellow when it is heated.
Reason The anionic sites occupied by unpaired electrons (due to F-centres).
110. Assertion Yb2+ is more stable in compare to Gd+2.
Reason The electronic configuration of GD is [Xe]4 f75d26s2.
111. Assertion Glucose does not gives 2, 4-DNP test.
Reason Glucose exists in cyclic hemiacetal form.
112. Assertion Phenol reacts with CH3I in presence of NaOH to form methoxybenzene.
Reason Phenoxide is better nucleophile than phenol.
113. Assertion Nylon-6 is condensation polymer.
Reason It is polymer of caprolactum.
114. Assertion Tert-butyl amine can be formed by Gabriel phthalimide synthesis.
Reason It follow SN1 mechanism.
115. Assertion Anhydrides are more reactive then ester for nucleophilic substitution.
Reason R.COO− is better leaving group than R−O−.
116. Assertion Propene reacts with H I in presence of peroxide give 1-iodopropane.
Reason 1° free radical is less stable than 2° free radical.
117. Assertion For liquid dishwashing non-ionic type of detergent are used.
Reason Remove grease and oil by micelle formation.
118. Assertion d5 configuration is more stable than d4.
Reason d5 has more exchange energy as compared to d4 because 10 and 6 exchanges are possible in d5 and d4 respectively.
119. Assertion The graph between pV v/s 1/V is a straight line.
Reason For adiabatic process, p ∝ 1/V
120. Assertion Tryptophan is an example of non-essential amino acids.
Reason The amino acids that are not synthesized in human body are non-essential aminoacids.
Part C : Biology
121. Which of the following is correct?
(a) Perigynous-Plum, peach, rose
(b) Epigynous-Guava and Cucumber
(c) Hypogynous-Mustard and rose
(d) Both (a) and (b)
122. Chimeric DNA is
(a) gene clone
(b) recombinant DNA
(c) transposon
(d) vector shuttle
123. Which of the following are homosporous?
(a) Salvinia, Equisetum
(b) Salvinia, Lycopodium
(c) Selaginella, Salvinia
(d) Lycopodium, Equisetum
124. What is the site of C3 cycle in C3 and C4 plants?
(a) In C3 plants-Mesophyll cell and in C4 plants-Bundle sheath cell
(b) In C3 plants-Bundle sheath cell and in C4 polants-Mesophyll cell
(c) In C4 plants-Bundle sheath cell and in C3 plants-Bundle sheath cell
(d) In C3 plants-Mesophyll cell and in C4 plants-Mesophyll cell
125. Which of the following represent zygomorphic symmetry?
(a) Canna, Mustard, Chilly, Datura
(b) Mustard, Canna, Pea, Datura
(c) Pea, Bean, Cassia, Gulmohar
(d) Pea, Bean, Canna, Chilly
126. In Ti-plasmid, which of the following is removed?
(a) Auxin gene
(b) Virulent gene
(c) Cytokinin gene
(d) Auxin and cytokinin gene
127. Which statement is correct?
(a) Mycoplasma is smallest and wall less living organism
(b) Influenza and herpes caused by virus having DNA and RNA
(c) Nostoc and Anabaena are important decomposer
(d) Methanogen are methane producing bacteria in wheat crop
128. The genetic codes of arginine are
(a) CGU, CGC, CGA
(b) CAU, CAC, CAA
(c) AGU, AGC, AAC
(d) GAU, GAC, GAA
129. King of spices is
(a) Brassica nigra
(b) Piper nigrum
(c) Piper longum
(d) Curcuma longa
130. ATP formation occurs through which of the following?
(a) Photophosphorylation
(b) Oxidative phosphorylation
(c) Substrate level phosphorylation
(d) All of the above
131. What are the requirements in tissue culture?
(a) Hormones like auxin, cytokinin, agar-agar
(b) Inorganic salt, vitamin, amino acid only
(c) Carbon source like sucrose only
(d) All of the above
132. Oxytocin and ADH are produced by hypothalamus and released from
(a) anterior pituitary
(b) posterior pituitary
(c) pineal gland
(d) thymus
133. Characteristic of female cockroach
(a) presence of anal style
(b) each ovary is made up ‘6’ ovarioles
(c) one pair spermatheca present and opens in genital chamber
(d) genital pouch is made up of 9th, 10th tergum and 9th sternum
134. Which of the following linkage is found in sucrose?
(a) 1, 2-glycosidic linkage
(b) 1, 4-glycosidic linkage
(c) 1, 3-glycosidic linkage
(d) 1, 1-glycosidic linkage
135. Which cell is found in mucus secreting organs?
(a) Goblet cells
(b) Paneth cells
(c) Oxyntic cells
(d) Peptic cells
136. Glucose or reacting with Benedict’s solution may give the following precipitates except
(a) violet precipitate
(b) orange red precipitate
(c) brick red precipitate
(d) green/yellow precipitate
137. Cervical vertebrae differ from the vertebrae in having
(a) spinous process
(b) centrum
(c) transverse process
(d) transverse foramen
138. Mark the incorrect statement for inbreeding.
(a) Inbreeding depression increases productivity
(b) Inbreeding depression can be overcome by outcrossing
(c) Produces purelines
(d) Increases homozygosity
139. Animal of which phylum have hooks and suckers and are endoparasite on other animals?
(a) Platyhelminthes
(b) Annelida
(c) Aschelminthes
(d) Arthropoda
140. Select the wrong statement from the following.
(a) The human genome contains 3164.7 million nucleotide bases
(b) Less than 10% of the genome codes for protein
(c) Repeated sequences make up very large portion of the human genome
(d) Chromosome 1 has most genes (2968) and Y has the fewest (231)
141. Protein on reaction with which yields Ruhemann’s purple?
(a) Ninhydrin
(b) Cu2+
(c) H2O2
(d) Benedict’s solution
142. Gene library or DNA library has the collection of
(a) DNA and RNA
(b) Any one type of gene of organism
(c) cDNA
(d) All possible genes are organisms
143. Which of the following are all nucleotides?
(a) Adenosine, Cytidilic acid, Cytosine
(b) Adenylic acid, Cytidilic acid, Guanylic acid
(c) Cytidine, Adenine, Adenylic acid
(d) Uracil, Thymidine, Thymidylic acid
144. Match the following columns and choose the correct option from the codes given below.
Column I Column II
(Substrate) (Enzyme)
(A) Ribonucleotide (i) Chitinase
(B) Chitin (ii) Cellulase
(C) Cellulose (iii) Ribonuclease
(a) A – (i), B – (ii), C – (iii)
(b) A – (iii), B – (i), C – (ii)
(c) A – (iii), B – (ii), C – (i)
(d) A – (ii), B – (i), C – (iii)
145. Match the following columns and choose the correct option from the codes given below.
Column I Column II
(A) Chrysophyte (i) Gonyaulax
(B) Dinoflagellate (ii) Euglena
(C) Euglenoids (iii) Diatom
(D) Slime moulds (iv) Plasmodium
(a) A – (i), B – (iii), C – (ii), D – (iv)
(b) A – (i), B – (iv), C – (ii), D – (iii)
(c) A – (iii), B – (ii), C – (iv), D – (i)
(d) A – (iii), B – (i), C – (ii), D – (iv)
146. Which of the following is incorrect?
(a) Mango and coconut are drupe fruit
(b) According to Euro Norm IV sulphur content for petrol is 50 ppm
(c) CO2 and other poisonous gases cause pollution
(d) Apple is a true fruit
147. The process of removal of anther from the flower bud before it dehisces is called as
(a) Emasculation
(b) Bagging
(c) Embryo rescue
(d) Budding
148. Match the following columns and choose the correct option from the codes given below.
Column I Column II
(A) Tap root (i) Sweet potato
(B) Adventitious root (ii) Turnip
(C) Stem (iii) Wheat
(D) Fibrous root (iv) Potato
(a) A – (i), B – (ii), C – (iii), D – (iv)
(b) A – (ii), B – (iii), C – (i), D – (iv)
(c) A – (ii), B – (i), C – (iv), D – (iii)
(d) A – (iv), B – (iii), C – (ii), D – (i)
149. Match the following columns and choose the correct option from the codes given below.
Column I Column II
(A) Pleiotropic (i) Both alleles express equally
(B) Co-dominance (ii) Change in nucleotides
(C) Epistasis (iii) One gene shows multiple phenotypic expression
(D) Mutation (iv) Non-allelic gene inheritance
(a) A – (i), B – (ii), C – (iii), D – (iv)
(b) A – (ii), B – (iii), C – (iv), D – (i)
(c) A – (iii), B – (i), C – (iv), D – (ii)
(d) A – (i), B – (iii), C – (iv), D – (ii)
150. Match the following columns and choose the correct option from the codes given below.
Column I Column II
(A) KC Mehta (i) Fluid mosaic model
(B) P. Maheswari (ii) First recombinant plasmid
(C) Cohen and Boyer (iii) Haploid culture
(D) Singer and Nicolson (iv) Rust disease
(a) A – (i), B – (iii), C – (ii), D – (iv)
(b) A – (iv), B – (iii), C – (ii), D – (i)
(c) A – (i), B – (ii), C – (iii), D – (iv)
(d) A – (ii), B – (iii), C – (iv), D – (i)
151. What is the function of Bowman’s capsule and glomerulus?
(a) Filtration of blood
(b) Reabsorption of ions from blood
(c) Reabsorption of hormones from blood
(d) Reabsorption of water from blood
152. Virus free plants can be formed by
(a) meristem culture
(b) callus culture
(c) somatic cell culture
(d) protoplast fusion
153. Immunity tolerance developed by
(a) interaction with the antigen
(b) giving antibodies
(c) present by birth
(d) giving antibiotics
154. Hairy root disease of dicot plants is caused by
(a) Agrobacterium tumefacians
(b) Agrobacterium rhizogene
(c) Bacillus thuringiensis
(d) Meloidogyne incognita
155. Choose the correct option.
(a) A. Testis-possesses 3-4 testicular lobule
(b) B. Seminal vesicle-storage of sperm
(c) C. Vas deferens-help in sperm transfer
(d) D. Prostate gland-secretes seminal fluid
156. Identify the given diagram of tissue performing secretion and absorption.
(a) Simple cuboidal epithelium
(b) Simple columnar epithelium
(c) Stratified cuboidal epithelium
(d) Stratified columnar epithelium
157. 
Identify A, B and C
(a) ‘A’ Elongation, ‘B’ Termination, ‘C’ Initiation
(b) ‘A’ Initiation, ‘B’ Termination, ‘C’ Elongation
(c) ‘A’ Initiation, ‘B’ Elongation, ‘C’ Termination
(d) ‘A’ Termination, ‘B’ Elongation, ‘C’ Initiation
158. 
Select the correct option w.r.t. age pyramids.
(a) A-Expanding, B-Stable, C-Declining
(b) A-Stable, B-Expanding, C-Declining
(c) A-Stable, B-Declining, C-Expanding
(d) A-Declining, B-Stable, C-Expanding
159. Blood group of the father is ‘A’ and blood group of mother of ‘B’. Then predict the blood group of the progeny
(a) A, AB
(b) A, B, AB, O
(c) B, AB
(d) O, A, B
160. It mitochondria is absent in mature RBC, what will be the source of energy?
(a) TCA
(b) ETS
(c) Link reaction
(d) Glycolysis
Directions for questions (161-180) In each of the following questions, a statement of Assertion is given followed by a corresponding statement of Reason just below it. Of the statements, mark the correct answer as
(a) If both Assertion and Reason are true and Reason is the correct explanation of Assertion
(b) If both Assertion and Reason are true, but Reason is not the correct explanation of Assertion
(c) If Assertion is true, but reason is false
(d) If both Assertion and Reason are false
161. Assertion Cholecystokinin is released by duodenum.
Reason It activates pepsinogen and bile juice.
bg_collapse view=”button-orange” color=”#4a4949″ icon=”arrow” expand_text=”Show Answer” collapse_text=”Hide Answer” ]
Answer: (c)
[/bg_collapse]
162. Assertion SA node malfunctioning leads to disturbance of heart rate.
Reason SA node is the pacemaker of heart producing electric impulse for heart contraction.
bg_collapse view=”button-orange” color=”#4a4949″ icon=”arrow” expand_text=”Show Answer” collapse_text=”Hide Answer” ]
Answer: (a)
[/bg_collapse]
163. Assertion Selaginella and Salvinia are homosporous.
Reason In pteridophyte, Lycopodium is precursor of seed habit.
bg_collapse view=”button-orange” color=”#4a4949″ icon=”arrow” expand_text=”Show Answer” collapse_text=”Hide Answer” ]
Answer: (d)
[/bg_collapse]
164. Assertion Gibberellin is useful in early seed production in conifers.
Reason Ethephon is responsible for early ripening in tomato and apple.
bg_collapse view=”button-orange” color=”#4a4949″ icon=”arrow” expand_text=”Show Answer” collapse_text=”Hide Answer” ]
Answer: (b)
[/bg_collapse]
165. Assertion Heterospory and retention of female gametophyte are responsible for origin of seed habit in
Reason Psilotum is a living fossil.
bg_collapse view=”button-orange” color=”#4a4949″ icon=”arrow” expand_text=”Show Answer” collapse_text=”Hide Answer” ]
Answer: (b)
[/bg_collapse]
166. Assertion Type-I diabetes is caused by destruction of β-cells of islets of Langerhans.
Reason Insulin can be taken as pills.
bg_collapse view=”button-orange” color=”#4a4949″ icon=”arrow” expand_text=”Show Answer” collapse_text=”Hide Answer” ]
Answer: (c)
[/bg_collapse]
167. Assertion Pituitary gland releases a hormone which is helpful in childbirth.
Reason Pituitary gland releases vasopressin and anti-diuretic hormone which helps in childbirth.
bg_collapse view=”button-orange” color=”#4a4949″ icon=”arrow” expand_text=”Show Answer” collapse_text=”Hide Answer” ]
Answer: (c)
[/bg_collapse]
168. Assertion Gastrin is a hormone that is released from the gastrointestinal tract and helps in digestion.
Reason It promotes secretion of HCl and trysinogen.
bg_collapse view=”button-orange” color=”#4a4949″ icon=”arrow” expand_text=”Show Answer” collapse_text=”Hide Answer” ]
Answer: (c)
[/bg_collapse]
169. Assertion Biofortified crop is a source of higher proteins, minerals and healtheier fats.
Reason Azolla is biofertiliser.
bg_collapse view=”button-orange” color=”#4a4949″ icon=”arrow” expand_text=”Show Answer” collapse_text=”Hide Answer” ]
Answer: (b)
[/bg_collapse]
170. Assertion Respiratory path way is considered as an amphibolic pathway.
Reason It involves both anabolism and catabolism.
bg_collapse view=”button-orange” color=”#4a4949″ icon=”arrow” expand_text=”Show Answer” collapse_text=”Hide Answer” ]
Answer: (a)
[/bg_collapse]
171. Assertion Down’s syndrome, Klinefelter’s syndrome and Turner’s syndrome are chromosomal disorders.
Reason In Klinefelter’s syndrome females are sterile.
bg_collapse view=”button-orange” color=”#4a4949″ icon=”arrow” expand_text=”Show Answer” collapse_text=”Hide Answer” ]
Answer: (c)
[/bg_collapse]
172. Assertion Deficiency of an element may lead to scurvy.
Reason Daily requirement of ascorbic acid is 5 mg/day.
bg_collapse view=”button-orange” color=”#4a4949″ icon=”arrow” expand_text=”Show Answer” collapse_text=”Hide Answer” ]
Answer: (d)
[/bg_collapse]
173. Assertion Cannabinoids are drugs of abuse.
Reason They affect cardiovascular system and central nervous system activity.
bg_collapse view=”button-orange” color=”#4a4949″ icon=”arrow” expand_text=”Show Answer” collapse_text=”Hide Answer” ]
Answer: (a)
[/bg_collapse]
174. Assertion Malpighian tubules are excretory organs in most of the insects.
Reason These help in excretion of urea and creatinine.
bg_collapse view=”button-orange” color=”#4a4949″ icon=”arrow” expand_text=”Show Answer” collapse_text=”Hide Answer” ]
Answer: (c)
[/bg_collapse]
175. Assertion In commensalism, one organism is benefitted and other is unaffected.
Reason Cattle egret bird and cattle is an example of commensalism.
bg_collapse view=”button-orange” color=”#4a4949″ icon=”arrow” expand_text=”Show Answer” collapse_text=”Hide Answer” ]
Answer: (b)
[/bg_collapse]
176. Assertion In C3 cycle, the first stable compound of 3C compound.
Reason In C4 plants, Calvin cycle is absent.
bg_collapse view=”button-orange” color=”#4a4949″ icon=”arrow” expand_text=”Show Answer” collapse_text=”Hide Answer” ]
Answer: (c)
[/bg_collapse]
177. Assertion In eukaryotes, transcription occurs in nucleus.
Reason In bacteria, transcription and translation occurs in cytoplasm.
bg_collapse view=”button-orange” color=”#4a4949″ icon=”arrow” expand_text=”Show Answer” collapse_text=”Hide Answer” ]
Answer: (b)
[/bg_collapse]
178. Assertion Biofortification is used to increase nutrient value of crops.
Reason Meristem culture is used to obtain virus resistant plants.
bg_collapse view=”button-orange” color=”#4a4949″ icon=”arrow” expand_text=”Show Answer” collapse_text=”Hide Answer” ]
Answer: (c)
[/bg_collapse]
179. Assertion Calcium required for skeletal muscle contraction.
Reason Calcium influx releases acetylcholine at neuromuscular junction.
bg_collapse view=”button-orange” color=”#4a4949″ icon=”arrow” expand_text=”Show Answer” collapse_text=”Hide Answer” ]
Answer: (c)
[/bg_collapse]
180. Assertion Parthenocarpy involves the formation of seedless fruit.
Reason Apomixis occurs without fertilization.
bg_collapse view=”button-orange” color=”#4a4949″ icon=”arrow” expand_text=”Show Answer” collapse_text=”Hide Answer” ]
Answer: (b)
[/bg_collapse]
Part D : GK & Aptitude & Logical Thinking
181. For how many seats does the parliament hold Elections?
(a) 540
(b) 541
(c) 542
(d) 543
182. Who is the present chief election commissioner?
(a) Misa Bharati
(b) Sunil Arora
(c) M. N. Singh
(d) Prashant kishore
183. Which country has not yet conducted anti-satellite missile test?
(a) India
(b) US
(c) Russia
(d) France
184. Arrange the cities from East to West
(I) Cairo (II) Tehran
(III) Triop (IV) Baghdad
(a) I, II, III, IV
(b) II, IV, I, III
(c) III, I, IV, II
(d) IV, III, II, I
185. What will be the next number in the series?
10, 9, 7, 4, ?
(a) 3
(b) 2
(c) 1
(d) 0
186. Ram is the brother of Seema and Ram is married to Radhika. Seema has two sons Raushan and Manu. What is Raushan to Radhika.
(a) Niece
(b) Nephew
(c) Cousin
(d) None of these
187. Which of the following states is odd one out in terms international border?
(a) Rajasthan
(b) Gujarat
(c) Punjab
(d) Himachal Pradesh
188. Neerav Modi is associated with
(a) Share Market
(b) Oil
(c) Diamond
(d) Gold
189. Which of the following van-diagram represents relations between them female : sister : : parents
190. Arrange the following states in decreasing order of their LOK SABHA seats.
(a) Madhya Pradesh > Maharashtra > Bihar > West Bengal
(b) Maharashtra > Bihar > West Bengal > Madhya Pradesh
(c) Madhya Pradesh > Maharashtra > Bihar > West Bengal
(d) Maharashtra > West Bengal > Bihar > Madhya Pradesh
191. Loksabha Elections 2019 have been conducted in how many phases?
(a) 5
(b) 6
(c) 7
(d) 8
192. In a shopping plaza, 20% discount is being offered on a product of Rs 2000/-. If the shop owner offer 10% additional discount on the actual/initial price at the time of billing, what will be the final price of the product?
(a) 1800
(b) 1600
(c) 1440
(d) 1400
193. Wiki Leaks founder Julian Assange was arrested recently from the embassy of
(a) France
(b) Italy
(c) Educator
(d) Dubai
194. Among the following rivers, which river has a different direction of flow?
(a) Godavari
(b) Kaveri
(c) Narmada
(d) Krishna
195. Who is the Governor of Reserve bank of India (RBI)
(a) C. Rangarajan
(b) Man Mohan Singh
(c) Shaktikanta Das
(d) Urjit Patel
196. Which agency conducted anti-satellite missile test recently?
(a) Research and Analysis Wing (RAW)
(b) Defence Research and Development Organization (DRDO)
(c) Indian Space Research Organization (ISRO)
(d) Indian Council of Agricultural
197. Find the odd one out with respect to Coastal Line of India.
(a) Karnataka
(b) Odisha
(c) Tamilnadu
(d) Andhra Pradesh
198. 3 Fruits Banana, Mango and Orange were available in the ratio 3 : 4 : 1. 3 Bananas are removed from the total available fruits and ratio of remaining fruits become 2 : 4 : 1. Find the total number of remaining fruits after removing 3 bananas?
(a) 21
(b) 20
(c) 19
(d) 18
199. “Kind of Good Times” is associated with.
(a) Neerav Modi
(b) Mehul Chauksi
(c) Gupta Brothers
(d) Vijay Mallya
200. Which aircraft were used in air strike in Balakot?
(a) Mig-21
(b) Mirage 2000
(c) F-16
(d) Mi-35
Latest Govt Job & Exam Updates: