JEE Main (AIEEE) 2004
Chemistry
1. Which of the following sets of quantum numbers is correct for an electron in 4f orbital ?
(1) n = 4, l = 3, , m = +4, s = +1/2
(2) n = 4, l = 4, m = −4, s = −1/2
(3) n = 4, l = 3, m = + 1, s = +1/2
(4) n = 3, l = 2, m = −2, s = +1/2
2. Consider the ground state of Cr atom (Z = 24). The numbers of electrons with the azimuthal quantum numbers, l = 1 and 2 are , respectively 3.
(1) 12 and 4
(2) 12 and 5
(3) 16 and 4
(4) 16 and 5
3. Which one of the following ions has the highest value of ionic radius?
(1) Li+
(2) B3+
(3) O2−
(4) F−
4. The wavelength of the radiation emitted, when in a hydrogen atom electron falls from infinity to stationary state 1, would be (Rydberg constant = 1.097 × 107 m−1)
(1) 91 nm
(2) 192 nm
(3) 406 nm
(4) 9.1 × 10−8 nm
5. The correct order of bond angles (smallest first) in H2S, NH3, BF3 and SiH4 is
(1) H2S < SiH4 < NH3 < BF3
(2) NH3 < H2S < SiH4 < BF3
(3) H2S < NH3 < SiH4 < BF3
(4) H2S < NH3 < BF3 < SiH4
6. Which one of the following sets of ions represents the collection of isoelectronic species?
(Atomic numbers F = 9, Cl = 17, Na = 11, Mg = 12, Al = 13, K = 19, Ca = 20, sc = 21)
(1) K+, Ca2+, Sc3+, Cl−
(2) Na+, Ca2+, Sc3+, F−
(3) K+, Cl−, Mg2+, Sc3+
(4) Na+, Mg2+, Al3+, Cl−
7. Among Al2O3, SiO2, P2O3 and SO2 the correct order of acid strength is
(1) SO2 < P2O3 < SiO2 < Al2O3
(2) SiO2 < SO2 < Al2O3 < P2O3
(3) Al2O3 < SiO2 < SO2 < P2O3
(4) Al2O3 < SiO2 < P2O3 < SO2
8. The bond order in NO is 2.5 while that in NO+ is 3. Which of the following statements is true for these two species?
(1) Bon length NO+ is greater than in NO
(2) Bond length in NO is greater than in NO+
(3) Bon length in NO+ is equal to that in NO
(4) Bond length is unpredictable
9. The formation of the oxide ion O2− (g) requires first an exothermic and then and endothermic step as shown below
O(g) + e− = O−(g); ∆H° = −142 kJ mol−1
O(g) − + e−= O2−(g); ∆H° = 844 kJ mol−1
(1) oxygen is more electronegative
(2) oxygen has high electron affinity
(3) O− ion will tend to resist the addition of another electron
(4) O− ion has comparatively larger size than oxygen atom
10. The states of hybridization of boron and oxygen atoms in boric acid (H3BO3) are respectively
(1) sp2 and sp2
(2) sp2 and sp3
(3) sp3 and sp2
(4) sp3 and sp3
11. Which one of the following has the regular tetrahedral structure?
(Atomic numbers B = 5, S = 16, Ni = 28, Xe = 54)
(1) XeF4
(2) SF4
(3) BF4−
(4) [Ni(CN)4]2−
12. Of the following outer electronic configurations of atoms, the highest oxidation state is achieved by which one of them?
(1) (n – 1)d8ns2
(2) (n – 1)d5ns1
(3) (n – 1)d3ns2
(4) (n – 1)d5ns2
13. As the temperature is raised from 20℃ to 40℃, the average kinetic energy of neon atoms changes by a factor of which of the following?
(1) 1/2
(2) ![]()
(3) 313/293
(4) 2
14. The maximum number of 90° angles between bond pair-bond pair of electrons is observed in
(1) dsp3 hybridization
(2) sp3d hybridization
(3) dsp2 hybridization
(4) sp3d2 hybridization
15. Which one of the following aqueous solutions will exhibit highest boiling point?
(1) 0.01 M Na2SO4
(2) 0.01 M KNO3
(3) 0.015 M urea
(4) 0.015 M glucose
16. Which among the following factors is the most important in making fluorine the strongest oxidizing agent?
(1) Electron affinity
(2) Ionization enthalpy
(3) Hydration enthalpy
(4) Bond dissociation energy
17. In van der Waal’s equation of state of the gas law, the constant ‘b’ is a measure of
(1) intermolecular repulsions
(2) intermolecular attraction
(3) volume occupied by the molecules
(4) intermolecular collisions per unit volume
18. The conjugate base of ![]() is
is
(1) PO43−
(2) P2O5
(3) H3PO4
(4) HPO42−
19. 6.02 × 1020 molecules of urea are present in 100 mL of its solution. The concentration of urea solution is
(Avogadro constant, NA = 6.02 × 1023 mol-1)
(1) 0.001 M
(2) 0.01 M
(3) 0.02 M
(4) 0.1 M
20. To neutralize completely 20 mL of 0.1 M aqueous solution of phosphorus acid (H3PO3), the volume of 0.1 M aqueous KOH solution required is
(1) 10 mL
(2) 20 mL
(3) 40 mL
(4) 60 mL
21. For which of the following parameters the structural isomers C2H5OH and CH3OCH3 would be expected to have the same values ?
(Assume ideal behaviour)
(1) Heat of vaporization
(2) Vapour pressure at the same temperature
(3) Boiling points
(4) Gaseous densities at the same temperature and pressure
22. Which of the following liquid pairs shows a positive deviation from Raoult’s law
(1) Water — Hydrochloric acid
(2) Benzene — methanol
(3) Water — nitric acid
(4) Acetone — chloroform
23. Which one of the following statement is false?
(1) Raoult’s law states that the vapour pressure of a component over a solution is proportional to its more fraction
(2) The osmotic pressure (π) of a solution is given by the equation π = MRT, where M is the molarity of solution
(3) The correct order of osmotic pressure of 0.01 M aqueous solution of each compound is BaCl2 > KCl > CH3COOH > sucrose
(4) Two sucrose solutions of same molality prepared in different solvents will have the same freezing point depression
24. An ideal gas expands in volume from 1 × 10−3 m3 to 1 × 10−2 m3 at 300 K against a constant pressure of 1 × 105 Nm−2. The work done is
(1) −900 J
(2) −900 kJ
(3) 270 kJ
(4) 900 kJ
25. In a hydrogen-oxygen fuel cell, combustion of hydrogen occurs to
(1) generate heat
(2) create potential difference between the two electrodes
(3) produce high purity water
(4) remove adsorbed oxygen from electrode surfaces
26. In a first order reaction, the concentration of the reactant, decreases from 0.8 M to 0.4 M in 15 min. The time taken for the concentration to change from 0.1 M to 0.025 M is
(1) 30 min
(2) 15 min
(3) 7.5 min
(4) 60 min
27. What is the equilibrium expression for the reaction
P4(s) + 5O2(g) ⇌ P4O10(s) ?
(1) 
(2) 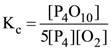
(3) Kc = [O2]5
(4) 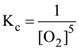
28. For the reaction,
CO(g) + Cl2(g) ⇌ COCl2(g), the Kp/Kc is equal to
(1) 1/RT
(2) RT
(3) √RT
(4) 1.0
29. The equilibrium constant for the reaction
N2(g) + O2(g) ⇌ 2NO(g)
At temperature T is 4 × 10−4. The value of Kc for the reaction
NO(g) ⇌ (1/2) N2(g) + (1/2) O2 (g) at the same temperature is
(1) 2.5 × 102
(2) 50
(3) 4 × 10−4
(4) 0.02
30. The rate equation for the reaction 2A + B →C is found to be, rate = k[A] [B]
The correct statement in relation to this reaction is that the
(1) unit of k must be s−1
(2) t1/2 is a constant
(3) rate of formation of C is twice the rate of disappearance of A
(4) value of k is independent of the initial concentrations of A and B
31. Consider the following E° values
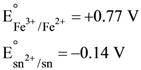
Under standard conditions the potential for the reaction
Sn(s) + 2Fe3+(aq) → 2Fe2+ (aq) + Sn2+ (aq) is
(1) 1.68 V
(2) 1.40 V
(3) 0.91 V
(4) 0.63 V
32. The molar solubility (in mol L−1) of a sparingly soluble salt M X4 is ‘S’. The corresponding solubility product is Ksp. S is given in terms of Ksp by the relation
(1) S = (Ksp/128)1/4
(2) S = (128 Ksp)1/4
(3) S = (256 Ksp)1/5
(4) S = (Ksp/256)1/5
33. The standard emf of a ell, involving one electron change is found to be 0.591 V at 25℃. The equilibrium constant of the reaction is (F = 96,500 C mol−1)
(1) 1.0 × 101
(2) 1.0 × 105
(3) 1.0 × 1010
(4) 1.0 × 1030
34. The enthalpies of combustion of carbon and carbon monoxide are −393.5 and −283 kJ mol−1 The enthalpy of formation of carbon monoxide per mole is
(1) 110.5 kJ
(2) 676.5 kJ
(3) −676.5 kJ
(4) −110.5 kJ
35. The limiting molar conductivities ⋀° for NaCl, KBr and KCl are 126, 152 and 150 S cm2 mol−1 The ⋀° for NaBr is
(1) 128 S cm2 mol−1
(2) 176 S cm2 mol−1
(3) 278 S cm2 mol−1
(4) 302 S cm2 mol−1
36. In a cell that utilizes the reaction
Zn(s) + 2H+(aq) → Zn2+(aq) + H2(g)
Addition of H2SO4 to cathode compartment, will
(1) lower the E and shift equilibrium to the left
(2) lower the E and shift the equilibrium to the right
(3) increase the E and shift the equilibrium to the right
(4) increase the E and shift the equilibrium to the left
37. Which one of the following statements regarding helium is incorrect?
(1) It is used to fill gas balloons instead of hydrogen because it is lighter and non-inflammable
(2) It is used as a cryogenic agent for carrying out experiments at low temperatures
(3) It is used to produce and sustain powerful superconducting magnets
(4) It is used in gas-cooled nuclear reactors
38. Identify the correct statement regarding enzymes
(1) Enzymes are specific biological catalysts that can normally function at very high temperatures (T ~ 1000 K)
(2) Enzymes are normally heterogeneous catalysts that are very specific in their action
(3) Enzymes are specific biological catalysts that cannot be poisoned
(4) Enzymes are specific biological catalysts that possess well defined active sites
39. One mole of magnesium nitride on the reaction with an excess of water gives
(1) One mole of ammonia
(2) one mole of nitric acid
(3) two moles of ammonia
(4) two moles of nitric acid
40. Which one of the following ores is best concentrated by froth-floatation method?
(1) Magnetite
(2) Cassiterite
(3) Galena
(4) Malachite
41. Beryllium and aluminum exhibit many properties which are similar. But, the two elements differ in
(1) exhibiting maximum covalency in compounds
(2) forming polymeric hydrides
(3) forming covalent halides
(4) exhibiting amphoteric nature in their oxides
42. Aluminium chloride exists as dimer, Al2Cl6 in solid state as well as in solution of non-polar solvents such as benzene. When dissolved in water, it gives
(1) Al3+ + 3Cl−
(2) [Al(H2O)6]3+ + 3Cl−
(3) [Al(OH)6]3− + 3HCl
(4) Al2O3 + 6HCl
43. The soldiers of Napolean army while at Alps during freezing winter suffered a serious problem as regards to the tin buttons of their uniforms. White metallic tin buttons got converted to grey powder. This transformation is related to
(1) a change in the crystalline structure of tin
(2) an interaction with nitrogen of the air at very low temperatures
(3) a change in the partial pressure of oxygen in the air
(4) an interaction with water vapour contained in the humid air
44. The ![]() values for Cr, Mn, Fe and Co are −0.41, +1.57, +0.77 and +1.97 V respectively. For which one of these metals the change in oxidation state from +2 to +3 is easiest?
values for Cr, Mn, Fe and Co are −0.41, +1.57, +0.77 and +1.97 V respectively. For which one of these metals the change in oxidation state from +2 to +3 is easiest?
(1) Cr
(2) Mn
(3) Fe
(4) Co
45. Excess of KI reacts with CuSO4 solution and then Na2S2O3 solution is added to it. Which of the statements is incorrect for this reaction?
(1) Cu2I2 is formed
(2) CuI2 is formed
(3) Na2S2O3 is oxidized
(4) Evolved I2 is reduced
46. Among the properties (A) reducing (B) oxidizing (C) complexing, the set of properties shown by CN− ion towards metal species is
(1) A, B
(2) B, C
(3) C, A
(4) A, B, C
47. The coordination number of a central metal atom in a complex is determined by
(1) the number of ligands around a metal ion bonded by sigma bonds
(2) the number of ligands around a metal ion bonded by pi-bonds
(3) the number of ligands around a metal ion bonded by sigma and pi-bonds both
(4) the number of only anionic ligands bonded to the metal ion
48. Which one of the following complexes is an outer orbital complex? (Atomic numbers Mn = 25, Fe = 26, Co = 27, Ni = 28)
(1) [Fe(CN)6]4−
(2) [Mn(CN)6]4−
(3) [Co(NH3)6]3+
(4) [Ni(NH3)6]2+
49. Coordination compounds have greater importance in biological systems. In this context which of the following statements is incorrect?
(1) Chlorophylls are green pigments in plants and contain calcium
(2) Haemoglobin is the red pigment of blood and contains iron
(3) Cyanocobalamin is vitamin B12 and contains cobalt
(4) Carboxypeptidase-A is an enzyme and contains zinc
50. Cerium (Z = 58) is an important member of the lanthandies. Which of the following statements about cerium is incorrect?
(1) The common oxidation states of cerium are +3 and +4
(2) The +3 oxidation state of cerium is more stable than the +4 oxidation state
(3) The +4 oxidation state of cerium is not known in solutions
(4) Cerium (IV) acts as an oxidizing agent
51. Which one of the following has largest number of isomers?
(R = alkyl group, en = ethylenediamine)
(1) [Ru(NH3)4Cl2]+
(2) [Co(NH3)5Cl]2+
(3) [Ir(PR3)2H(CO)]2+
(4) [Co(en)2Cl2]+
52. The correct order of magnetic moments (spin only values in BM) among the following is
(Atomic numbers Mn = 25, Fe = 26, Co= 27)
(1) [MnCl4]2− > [CoCl4] 2− > [Fe(CN)6]4−
(2) [MnCl4] 2− > [Fe (CN)6] 4− > [CoCl4]2−
(3) [Fe (CN)6]4− > [MnCl4]2− > [CoCl4]2−
(4) [Fe (CN)6]4− > [CoCl4]2− > [MnCl4]2−
53. Consider the following nuclear reactions
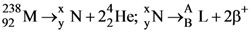
The number of neutrons in the element L is
(1) 142
(2) 144
(3) 140
(4) 146
54. The half-life of a radioisotope is four hours. If the initial mass of the isotope was 200 g, the mass remaining after 24 h undecayed is
(1) 1.042 g
(2) 2.084 g
(3) 3.125 g
(4) 4.167 g
55. The compound formed in the positive test for nitrogen with the Lassaigne solution of an organic compound is
(1) Fe4[Fe(CN)6]3
(2) Na3[Fe(CN)6]
(3) Fe(CN)3
(4) Na4[Fe(CN)5NOS]
56. The ammonia evolved from the treatment of 0.30 g of an organic compound for the estimation of nitrogen was passed in 100 mL of 0.1 M sulphuric acid. The excess of acid required 20 mL of 0.5 M sodium hydroxide solution for complete neutralization. The organic compound is
(1) acetamide
(2) benzamide
(3) urea
(4) thiourea
57. Which one of the following has the minimum boiling point?
(1) n-butane
(2) 1-butyne
(3) 1-butene
(4) Isobutene
58. The IUPAC name of the compound
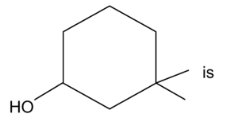
(1) 3,3-dimthyl-1-hydroxy cyclohexane
(2) 1,1-dimethyl-3-hydroxy cyclohexane
(3) 3,3-dimethyl-1-cyclohexanol
(4) 1,1-dimethyl-3-cyclohexanol
59. Which one of the following does not have sp2 hybridized carbon?
(1) Acetone
(2) Acetic acid
(3) Acetonitrile
(4) Acetamide
60. Which one of the following will have a meso-isomer also?
(1) 2-chlorobutane
(2) 2,3-dichlorobutane
(3) 2,3-dichloropentane
(4) 2-hydroxypropanoic acid
61. Rate of the reaction
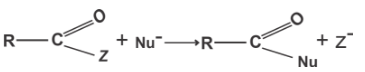
is fastest when Z is
(1) Cl
(2) NH2
(3) OC2H5
(4) OCOCH3
62. Amongst the following compounds, the optically active alkane having lowest molecular mass is
(1) CH3 – CH2 – CH2 – CH3
(2) 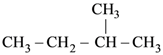
(3) 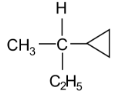
(4) CH3 – CH2 – C ≡ CH
63. Consider the acidity of the carboxylic acids
(i) PhCOOH (ii) o-NO2C6H4COOH
(iii) p-NO2C6H4COOH (iv) m-NO2C6H4COOH
(1) i > ii > iii > iv
(2) ii > iv > iii > i
(3) ii > iv > i > iii
(4) ii > iii > iv > i
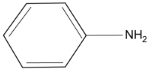
64. Which of the following is the strongest base ?
(1) 
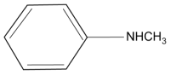
(2) 
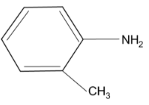
(3) 
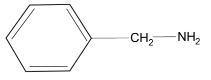
(4) 
65. Which base is represent in RNA but not in DNA?
(1) Uracil
(2) Cytosine
(3) Guanine
(4) Thymine
66. The compound formed on heating chlorobenzene with chloral in the presence of concentrated sulphuric acid is
(1) gammexane
(2) DDT
(3) Freon
(4) hexachloroethane
67. On mixing ethyl acetate aqueous sodium chloride, the composition of the resultant solution is
(1) CH3COOC2H5 + NaCl
(2) CH3COONa + C2H5OH
(3) CH3COCl + C2H5OH + NaOH
(4) CH3Cl + C2H5COONa
68. Acetyl bromide reacts with excess of CH3MgI followed by treatment with a saturated solution of NH4Cl gives
(1) acetone
(2) acetamide
(3) 2-methyl-2-propanol
(4) acetyl iodide
69. Which one of the following is reduced with zinc and hydrochloric acid to give the corresponding hydrocarbon?
(1) Ethyl acetate
(2) Acetic acid
(3) Acetamide
(4) Butan-2-one
70. Which one of the following undergoes reaction with 50% sodium hydroxide solution to give the corresponding alcohol and acid?
(1) Phenol
(2) Benzaldehyde
(3) Butanal
(4) Benzoic acid
71. Among the following compounds which can be dehydrated very easily?
(1) CH3CH2CH2CH2CH2OH
(2) 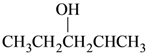
(3) 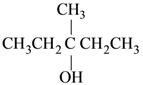
(4) 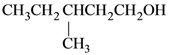
72. Which of the following compounds is not chiral?
(1) 1-chloropentane
(2) 2-chloropentane
(3) 1-chloro-2-methyl pentane
(4) 3-chloro-2-methyl pentane
73. Insulin production and its action in human body are responsible for the level of diabetes. The compound belongs to which of the following categories?
(1) A co-enzyme
(2) A hormone
(3) An enzyme
(4) An antibiotic
74. The smog is essentially caused by the presence of
(1) O2 and O3
(2) O2 and N2
(3) oxides of sulphur and nitrogen
(4) O3 and N2
75. What type of crystal defect is indicated in the diagram below?
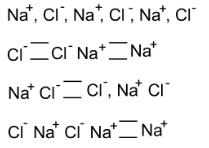
(1) Frenkel defect
(2) Schottky defect
(3) Interstitial defect
(4) Frenkel and Schottky defects
Latest Govt Job & Exam Updates: