Solved Paper -2015
VIT
Engineering Entrance Exam
Chemistry
1. Gaseous benzene reacts with hydrogen gas in presence of a nickel catalyst to form gaseous cyclohexane according to the reaction,
C6H6(g) + 3H2(g) → C6H12(g)
A mixture of C6H6 and excess H2 has pressure of 60 mm of Hg in an unknown volume. After the gas had been passed over a nickel catalyst and all the benzene converted to cyclohexane, the pressure of the gas was 30 mm Hg in the same volume at the same temperature. The fraction of C6H6 (by volume) present in the original volume is
(a) 1/3
(b) 1/4
(c) 1/5
(d) 1/6
2. An alloy of copper, silver and gold is found to have copper atom constituting the ccp lattice. If silver atom occupy the edge centres and gold atom is present at body centred, the alloy has formula
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
3. Given, ∆G° = −nFE°cell and ∆G° = −RT ln k.. The value of n = 1 will be given by the slope of which line in the figure
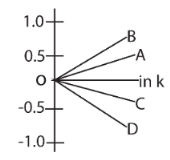
(a) OA
(b) OB
(c) OC
(d) OD
4. The false statements among the following are
I. A primary carbocation is less stable than a tertiary carbocation.
II. A secondary propyl carbocation is less stable than allyl carbocation.
III. A tertiary free radical is more stable than a primary free radical.
IV. Isopropyl carbanion is more stable than ethyl carbanion.
(a) I and II
(b) II and III
(c) I and IV
(d) II and IV
5. A colourless water soluble solid A on heating gives equimolar quantities of B and C. B given dense white fumes with HCl and C does to with NH3. B gives brown precipitate with Nessler’s reagent and C gives white precipitate with nitrates of Ag+, Pb+ and Hg+ A is
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
6. The IUPAC name of 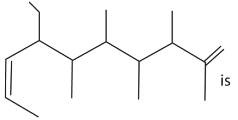
(a) 4-ethyl-5, 6 7, 9-tetramethyldeca-2, 9-diene
(b) 7-ethyl-2, 4, 5, 6-tetramethyldeca-1, 8-diene
(c) 7-ethyl-2, 4, 5, 6-tetramethyldeca-1, 7-diene
(d) 7-(1-propenyl)-2, 3 4, 5-tetramethyl non-1-ene
7. Caffeine has a molecular weight of 194 u. If it contains 28.9% by mass of nitrogen, number of atom of nitrogen in one molecular of caffeine is
(a) 4
(b) 6
(c) 2
(d) 3
8. A compound X on heating gives a colourless gas. The residue is dissolved in water to obtain Y. Excess CO2 is passed through aqueous solution of Y when Z is formed. Z on gentle heating gives back X. The compound X is
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
9. Which two sets of reactants best represents the amphoteric character of Zn(OH)2?
Set I Zn(OH)2 (s) and ![]()
Set II Zn(OH)2(s) and H2O (l)
Set III Zn(OH)2(s) and H+(aq)
Set IV Zn(OH)2(s) and NH3 (aq)
(a) III and II
(b) I and III
(c) IV and I
(d) II and IV
10. ![]() A and B respectively are
A and B respectively are
(a) 
(b) 
(c) 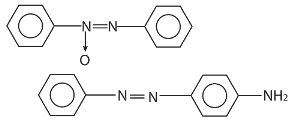
(d) None of the above
11. Point out incorrect stability order
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
12. Consider the following changes
M(s) → M(g) ….(1)
M(g) → M2+(g) + 2e− …..(2)
M(g) → M+(g) + e− ….(3)
M+(g) → M2+(g) + e− ….(4)
M(g) → M2+(g) + 2e− ….(5)
The second ionization energy of M could be determined from the energy values associated with
(a) 1 + 2 + 4
(b) 2 + 3 – 4
(c) 1 + 5 – 3
(d) 5 – 3
13. In benzene, the triple bond consists of
(a) one sp-sp sigma bond and two p-p pi bonds
(b) two sp-sp sigma bonds and one p-p pi bond
(c) one ![]() sigma bond, one p-p pi bond
sigma bond, one p-p pi bond
(d) one ![]() sigma bond, one
sigma bond, one ![]() pi bond and one p-p pi bond
pi bond and one p-p pi bond
14. In keto-enol tautomerism of dicarbonyl compounds, the enol-form is preferred in contrast to the keto-from, this is due to
(a) presence of carbonyl group on each side of ![]() group
group
(b) resonance stabilization of enol form
(c) presence of methylene group
(d) rapid chemical exchange
15. An organic compound having carbon, hydrogen and sulphur contains 4% of sulphur. The minimum molecular weight of the compound is
(a) 200
(b) 400
(c) 600
(d) 800
16. Which one of the following is a case of negative adsorption?
(a) Acetic acid solution in contact with animal charcoal.
(b) Dilute KCl solution in contact with blood charcoal.
(c) Concentration KCl solution in contact with blood charcoal.
(d) H2 gas in contact with charcoal at 300 K.
17. The concentrations of the reactant A in the reaction A → B at different times are given below

The rate constant of the reaction according to the correct order of reaction is
(a) 0.001 M/min
(b) 0.001 min−1
(c) 0.001 min/M
(d) 0.001 M−1 min−1
18. The ratio of slopes of Kmax vs V and V0 vs ν curves in the photoelectric effects gives (ν = frequency, Kmax = maximum kinetic energy, ν0 = stopping potential)
(a) the ratio of Planck’s constant of electronic charge
(b) work function
(c) Planck’s constant
(d) charge of electron
19. With excess of water, both P2O5 and PCl5 give
(a) H3PO3
(b) H3PO2
(c) H3PO4
(d) H4PO7
20. The dissolution of Al(OH)3 by a solution of NaOH results in the formation of
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
21. Which of the following does not exist?
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
22. If the ionization energy and electron affinity of an element are 275 and 86 kcal mol−1 respectively, then the electronegativity of the element on the Mulliken scale is
(a) 2.8
(b) 0.0
(c) 4.0
(d) 2.6
23. Which of the following sets of reactants is used for preparation of paracetamol from phenol?
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
24. A certain compound gives negative test with ninhydrin and positive test with Benedict’s solution. The compound is
(a) a protein
(b) a monosaccharide
(c) a lipid
(d) an amino acid
25. Super glue or crazy glue is
(a) poly(methyl methacrylate)
(b) poly (ethyl acrylate)
(c) poly (methyl α-cyanoacrylate)
(d) Poly (ethyl methacrylate)
26. 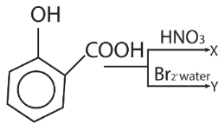
X and Y respectively are
(a) picric acid, 2, 4, 6-tribromophenol
(b) 5-nitrophenol acid, 5-bromosalicyclic acid
(c) o-nitrophenol, o-bromophenol
(d) 3, 5-dinitrosalicylica acid, 3, 5-dibromosalicylic acid
27. In the cannizzaro reaction given below
![]() the slowest step is
the slowest step is
(a) the attack of ![]() at the carbonyl group
at the carbonyl group
(b) the transfer of hydride ion to the carbonyl group
(c) the abstraction of proton from the carboxylic acid
(d) the deprotonation of ![]()
28. The reaction of 1-bromo-3-chlorocyclobutane with metallic sodium in dioxane under reflux conditions gives
(a) 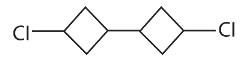
(b) 
(c) 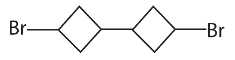
(d) 
29. Identify Z in the following reaction sequence
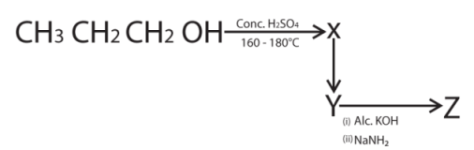
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
30. Which of the following reactions is sued be prepare isobutene?
(a) Wurtz reaction of C2H5Br
(b) Hydrolysis of n-butylmagnesium iodide
(c) Reduction of propanol with red phosphorus and HI
(d) Decarboxylation of 3-methylbutanoic acid
31. When copper is treated with a certain concentration of nitric acid, nitric oxide and nitrogen dioxide are liberated in equal volumes according to the equation,
xCu + yHNO3 → Cu(NO3)2+NO + NO2 + H2O The coefficients of x and y are respectively
(a) 2 and 3
(b) 2 and 6
(c) 1 and 3
(d) 3 and 8
32. A saturated solution of H2S in 0.1 M HCl at 25℃ contains S2− ion concentration of 10−23 mol L−1. The solubility product of some sulphides are Cus = 10−44, FeS = 10−14, MnS = 10−15, CdS = 10−25. If 0.01 M solution of these salts in 1M HCl are saturated with H2S, which of these will be precipitated?
(a) All
(b) All except MnS
(c) All except MnS and FeS
(d) Only CuS
33. Consider the water gas equilibrium reaction,
C(s) + H2O(g) ⇌ CO(g) + H2(g)
Which of the following statements is true at equilibrium?
(a) If the amount of C(s) is increased, less water would be formed
(b) If the amount of C(s) is increased, more would be formed
(c) If the pressure on the system is increased by halving the volume,, more water would be formed
(d) If the pressure on the system is increased by halving the volume, more CO and H2 would be formed
34. The chemical composition of slag formed during the smelting process in the extraction of copper is
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
35. XCl2 (excess) + YCl2 → X Cl4 + Y↓
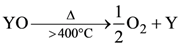
Ore of Y would be,
(a) siderite
(b) malachite
(c) hornsilver
(d) cinnabar
36. For the given reaction,
H2(g) + Cl2(g) → 2H+ (aq) + 2Cl− (aq); ∆G° = −262.4 kJ
The value of free energy of formation (∆G°f) for the ion Cl−1 (aq), therefore will be
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
37. The molarity of NO3− in the solution after 2L of 3M AgNO3 is mixed with 3 L of 1 M BaCl2 is
(a) 1.2 M
(b) 1.8 M
(c) 0.5 M
(d) 0.4 M
38. Amongest ![]() and
and ![]() the non-planar species are
the non-planar species are
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
39. 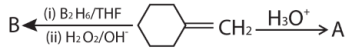
A and B respectively are
(a) ![]()
(b) 
(c) 
(d) 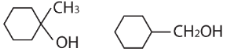
40. A certain metal when irradiated by light (r = 3.2 × 1016 Hz) emits photoelectrons with twice kinetic energy as did photoelectrons when the same metal is irradiated by light (r = 2.0 × 1016 Hz). The ν0 of metal is
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
Latest Govt Job & Exam Updates: