Model Question Paper For SRMJEEE
B.Tech and Health Sciences UG programs
Part-1 – Physics
1. The dimensional formula for impulse is
(a) MLT−1
(b) ML2T−1
(c) ML2T−2
(d) ML0T−2
2. The mean time period of a simple pendulum is 1.92 s. Mean absolute error in the time period is 0.05 s. To express the maximum estimate of error, the time period should be written as:
(a) T = (1.92 ± 0.001)s
(b) T = (1.92 ± 0.25)s
(c) T = (1.92 ± 0.05)s
(d) T = (1.92 ± 0.10)s
3. A motor boat moves at a steady speed of 8m/s. If the water resistance to the motion of the boat is 2000W, calculate the power of the engine.
(a) 16000 W
(b) 1600 W
(c) 160 W
(d) 16 W
4. An aeroplane travelling at a speed of 500 kmph tilts at an angle of 30o as it makes a turn. What is the radius of the curve?
(a) 341 km
(b) 3.41 km
(c) 0.341 km
(d) 34.1 km
5. A body of mass 500gm is tied to one end of a string of length 1m and is whirled round in a horizontal circle making 2 revolutions per second. Calculate the tension in the string.
(a) 78.88 N
(b) 7.888 N
(c) 0.7888 N
(d) 7888 N
6. A bullet of mass 10gm moving with a speed of 500 m/s gets embedded in a tree after penetrating 5cm into it. Calculate the average retarding force exerted by the wood on the bullet and the work done by the wood in bringing the bullet to stop.
(a) 25 N, 12.50 joule
(b) 250 N, 1250 joule
(c) 25 KN, 1.250 joule
(d) 25 KN, 1250 joule
7. The acceleration due to gravity g on earth in 9.8 ms−2. What would be the value of g for a planet whose size is the same as that of earth but the density in twice that of earth?
(a) 19.6 ms−2
(b) 9.8 ms−2
(c) 4.9 ms−2
(d) 2.45 ms−2
8. When a capillary tube of radius r is immersed in a liquid of density ρ the liquid rises to a height h in it. If m is the mass of the liquid in the capillary tube, the potential energy of this mass of the liquid in the tube is
(a) ![]()
(b) ![]()
(c) mgh
(d) 2 mgh
9. In which one of the following cases will the liquid flow in a pipe be most stream lined?
(a) Liquid of high viscosity and high density flowing through a pipe of small radius.
(b) Liquid of high viscosity and low density flowing through a pipe of small radius.
(c) Liquid of low viscosity and low density flowing through a pipe of large radius
(d) Liquid of low viscosity and high density flowing through a pipe of large radius
10. A stone is dropped into a lake by a person from a 500 m high tower. He would hear the sound of after approximately.
(a) 10 s
(b) 11.5 s
(c) 14 s
(d) 21 s
11. For the same pressure and density, the speed of sound is highest in a
(a) Monoatomic gas
(b) Diatomic gas
(c) Triatomic gas
(d) Polyatomic gas
12. The number of Beats produced / sec by the vibrations x1 = a sin 320 π t & x2 = a sin 326 πt is
(a) 6
(b) 4
(c) 3
(d) 2
13. Determine the volume occupied by 3.2 grams of oxygen at 76 cm of Hg and 27℃. (R = 8.314 × 107 ergs/ mol-k)
(a) 2.461 cm3
(b) 2461 cm3
(c) 246.1 cm3
(d) 24.61 cm3
14. Calculate the universal gas constant for one gram molecule of gas.
(a) 8.31 J/mol-k
(b) 83.1 J/mol-k
(c) 8.31 × 10−7 J/mol-k
(d) 83.1 × 10−7 J/mol-k
15. Number of molecules per unit volume of an ideal gas is ___________
(a) PRT
(b) PN/RT
(c) PT/RN
(d) RN/KT
16. Calculate the temperature at which the RMS velocity of a hydrogen molecule will be equal to the orbital velocity of earth’s satellite (i.e., 8 km/s)
(a) 514 × 103 K
(b) 5.14 × 103 K
(c) 51.4 × 103 K
(d) 514 K
17. Ordinary light is
(a) plane polarised
(b) circularly polarised
(c) alliptically polarised
(d) unpolarised
18. The force between two bar magnets whose centers are d metres apart is 4.8N. When the separation is made 2d the force is reduced to
(a) 2.4 N
(b) 1.2 N
(c) 0.6 N
(d) 0.3 N
19. In Young’s double slit experiment, the seventh maxima with light of wavelength λ1 is at d1 and the same maxima with same light of wavelength λ2 is at d2, then d1/d2 equals,
(a) λ1/ λ2
(b) λ2/ λ1
(c) λ12/ λ22
(d) λ22/ λ12
20. Which of the following undergo maximum diffraction?
(a) radio waves
(b) α-rays
(c) x-rays
(d) light waves
21. Electric flux in an electric field ![]() through area
through area ![]() is given by
is given by
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
22. A soap bubble is charged to a potential of 16V. Its radius is then doubled. The potential of the bubble now will be:
(a) 16 V
(b) 8 V
(c) 4 V
(d) 2 V
23. A bar magnet of magnetic moment pm is divided into two equal parts by cutting it perpendicular to its length. The magnetic moment of either piece will be:
(a) zero
(b) Pm/2
(c) Pm
(d) 2 Pm
24. What is the angle of dip at the magnetic poles?
(a) 0°
(b) 30°
(c) 45°
(d) 90°
25. What determines the penetrating power of X-rays?
(a) velocity
(b) frequency
(c) intensity
(d) density
26. A source of light is placed at a distance x from a photocell and the cut off potential is V. If the distance of the same source from the photocell is doubled, then cut off potential will be:
(a) V/4
(b) V/2
(c) V
(d) 2V
27. The orbital radius of the electron in the hydrogen atom changes from r to 4r. The energy of the orbital electron will change from E to:
(a) 4E
(b) 2E
(c) E/2
(d) E/4
28. The mass of photon at rest is:
(a) zero
(b) 1.67 × 1035 kg
(c) 1 amu
(d) 9 × 1031 kg
29. An electron and a proton possess same kinetic energy. Then ![]()
(a) 1
(b) 1840
(c) ![]()
(d) ![]()
30. What amount of energy is released in the fission of 92U235?
(a) 20 eV
(b) 200 eV
(c) 200 KeV
(d) 200 MeV
31. The helium atom does not contain
(a) 2 protons
(b) 2 electrons
(c) 2 neutrons
(d) 6 nucleons
32. A radioactive substance has half life of four months. Three fourth of the substance will decay in
(a) 3 months
(b) 4 months
(c) 12 months
(d) 8 months
33. NOR gate is a combination of
(a) OR gate and NOT gate
(b) Or gate and AND gate
(c) OR gate and OR gate
(d) AND gate only
34. The only function of a NOT gate is to
(a) stop a signal
(b) recomplement a signal
(c) invert an input signal
(d) act as a universal gate
35. When two semiconductors of p-type and N-type are brought in contact with each other the PN junction formed behaves like
(a) an oscillator
(b) a condenser
(c) an amplifier
(d) a conductor
Part 2 – Chemistry
36. Azidothymidine drug is used for treating ________ patients
(a) Diabetes
(b) AIDS
(c) Jaundice
(d) Tuberculosis
37. Which of the following weight the least?
(a) 2.0 gram mole of CO2
(b) 0.1 mole of sucrose (C12H22O11)
(c) 1 gram atom of calcium
(d) 1.5 mole of water
38. What is the value of gas constant R in Jmol−1 K−1
(a) 82.1
(b) 8.314 × 102
(c) 8.314
(d) 0.0821
39. Which process can be explained by the kinetic-molecular theory?
(a) combustion
(b) oxidation
(c) condensation
(d) displacement reaction
40. Which is an example of effusion?
(a) air slowly escaping from a pinhole in a tire
(b) the aroma of a cooling pie spreading across a room
(c) helium dispersing in to a room after a ballon pops
(d) oxygen and gasoline fumes mixing in an automobile carburetor
41. In each period, the strongest oxidising behaviour is of a/an
(a) Alkali metal
(b) Noble gas
(c) Halogens
(d) Chalcogen
42. The most electronegative and electropositive elements of the first period is/are
(a) H and He
(b) Na and Cl
(c) Li and F
(d) H and H
43. Beryllium has diagonal relationship with
(a) Li
(b) B
(c) Na
(d) Al
44. Mean distance between atoms is in the range of
(a) 25 nm
(b) 2.5 nm
(c) 0.25 nm
(d) 0.025 nm
45. What is the meaning of the word ‘atom’?
(a) dividable
(b) invisible
(c) hard particles
(d) not able to be divided
46. Dipole moment of BeF2 is
(a) very low
(b) very high
(c) zero
(d) not definite
47. Which molecule has the largest dipole moment?
(a) HCl
(b) HI
(c) HBr
(d) HF
48. C (diamond) → C (graphite) ∆H = − This shows that
(a) Graphite is more stable than diamond
(b) Diamond is more stable than graphite
(c) Both are equally stable
(d) Stability cannot be predicted
49. When Fe(s) is dissolved in aqueous hydrocholoric acid in a closed vessel, the work done is
(a) zero
(b) infinity
(c) extensive
(d) intensive
50. The sign convention for work done by the system is ______
(a) zero
(b) positive
(c) negative
(d) either positive or negative
51. In the solubility expression p = KH the term ‘KH’ infers
(a) Dalton’s constant
(b) Henry’s law constant
(c) Boltzmann constant
(d) Partial pressure of H2
52. The molal boiling point elevation constant for water is 0.510 K mol−1 The boiling point of a solution made by dissolving 6.0g urea in 200g water is
(a) 100.255℃
(b) 100℃
(c) 0.255℃
(d) 99.1℃
53. What is the conjugate base of OH−?
(a) O2−
(b) O−
(c) H2O
(d) O2
54. The reason for addition of catalyst in a reversible reaction at the beginning is
(a) to get the max concentration of reactants
(b) to increase the speed of attainment of equilibrium
(c) to alter the product concentration
(d) favour of forward reaction
55. The chemical formula of rust is
(a) Fe2O3 ∙ xH2O
(b) FeO
(c) Fe(OH)2
(d) FeO2 ∙ H2O
56. The sum of the powers of the exponentials to which each concentration term is raised is defined as
(a) rate of the reaction
(b) molecularity of the reaction
(c) order of the reaction
(d) half life period
57. A reaction was found to be second order with respect to the concentration of carbonmonoxide. If the concentration of carbonmonoxide is doubled with everything else kept the constant, the rate of reaction will
(a) increase by a factor of 4
(b) double
(c) remain unchanged
(d) triple
58. In molisch test the presence of carbohydrates is confirmed by the formation of
(a) yellow precipitate
(b) scarlet red solution
(c) greenish yellow fluorescence
(d) red ring at the junction of two layers of liquids
59. The molecular mass of compound A is 168 g/mol. Its empirical formula is C4H4 Find its molecular formula
(a) C4H4S
(b) C2H2S
(c) C8H8S2
(d) C2H4S
60. The isomerism exhibited by CH3CH2OCH2CH3 and CH3CH2CH2CH2OH is
(a) Structural isomerism
(b) Position Isomerism
(c) Optical isomerism
(d) Functional isomerism
61. A bond that undergoes heterolytic cleanage most readily is
(a) C-C
(b) O-H
(c) C-O
(d) C-H
62. The major poroduct obtained when 3-phenyl propene reacts with HBr
(a) 
(b) C6H5 – CH2 – CH2 – CH2 – Br
(c) 
(d) 
63. The reactions of alkenes are mainly
(a) Nucleophilic addition
(b) Nucleophilic substitution
(c) Free radical reactions
(d) Electrophilic additions
64. 
(a) Benzene
(b) Phenol
(c) Benzoic acid
(d) Salicylic acid
65. ![]() . Y is
. Y is
(a) acid
(b) aldehyde
(c) kitone
(d) ethanol
66. A  A is
A is
(a) CH3 – NH – CH3
(b) (CH3CH2)2 NH
(c) (CH3CH2) N–CH3
(d) CH3CH2CH2NHCH3
67. Schiffs base is formed when aniline is heated with
(a) C6H5CHO
(b) C6H5CH2OH
(c) C6H5CH2Cl
(d) C6H6
68. Styrene dissolves well in
(a) Water
(b) Benzene
(c) Methanol
(d) Ethylacetate
69. Fissuring at corners of mouth and lips due to the deficiency of Vitamin B2 is called
(a) Pernicious anaemia
(b) Xerophthalmia
(c) Cheilosis
(d) Rickets
70. _____________ is the first artificial sweetening agent discovered.
(a) sucrose
(b) fructose
(c) glucose
(d) saccharine
Part 3 – Mathematics
71. The domain of definition of the function  is
is
(a) (1, 2)
(b) (−10) ∪ (1, 2)
(c) (1, 2) ∪ (2, ∞)
(d) (−1, 1) ∪ (1, 2) ∪ (2, ∞)
72. What is the modulus and the principal value of the argument (− π < θ < π) of 1 + √2 + i
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
73. The conjugate of a complex number is ![]() . Then the complex number is
. Then the complex number is
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
74. Let A be 2 × 2 matrix with real entries. Let 3 be 2 × 2 identity matrix. Remote by tr(A) the sum of the diagonal entries of A. Assume that A2 = 1
Statement 1 : If A ≠ 1 and A ≠ −1 then det A = −1
Statement 2 : If A ≠ 1 and A ≠ −1 then tr (A) ≠ 0
(a) Statement 1 is false, statement 2 is true
(b) Statement 1 is true, statement 2 is true, statement 2 is a correct explanation for statement 1
(c) Statement 1 is true, statement 2 is true, statement 2 is not a connect explanation for statement 1
(d) Statement 1 is true, statement 2 is false
75. In a third order square matrix (aij) it is given that aij = 2i – 3j then the matrix is given by
(a) 
(b) 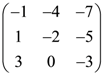
(c) 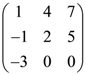
(d) 
76. If  and
and  then x =
then x =
(a) 
(b) 
(c) 
(d) 
77. If  and
and  then x =
then x =
(a) 
(b) 
(c) 
(d) 
78. If A is a square matrix of order 3 then the true statement is
(a) det(−A) = −det A
(b) det A = 0
(c) det (A + I) = I + det A
(d) det (2A) = 2 det A
79. For the equation 3x2 + px + 3 = 0, p > 0, if one of the roots is square of the other, then p is equal to
(a) 1/3
(b) 1
(c) 3
(d) 2/3
80. The least value of n for which (n – 1 C4 + n – 1 C3) > nC3 is
(a) 7
(b) 9
(c) 10
(d) 8
81. By induction, value of expression cos θ cos 2θ cos 4θ ….. cos (2n – 1 θ) is equal to
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
82. If a, b, c, d ∈ ℝ+ and a, b, c, d are in H.P then
(a) a + d > b + c
(b) a + c > b + d
(c) a + b > c + d
(d) a – b > c – d
83. The 99th term of the sequence 2, 7, 14, 23, 34, …… is
(a) 9998
(b) 9999
(c) 10000
(d) 10001
84. If  is continuous at x = 0, then the value of k is
is continuous at x = 0, then the value of k is
(a) b + a
(b) b –2a
(c) 2a – b
(d) 2a + b
85. The range of the function  is
is
(a) (−∞, ∞)
(b) [−2, 2]
(c) [−1, 1]
(d) 
86. For the curve x = t2 – 1, y = t2 – t, the tangent line is perpendicular to x-axis where
(a) t = 0
(b) t → ∞
(c) t = 1/√3
(d) t = −1/√3
87. Rolle’s theorem holds for the function x3 + bx2 + cx, 1 ≤ x ≤ 2 at the point 4/3, the value of b and c are
(a) 8, −5
(b) −5, 8
(c) 5, −8
(d) −5, −8
88. 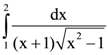 is equal to
is equal to
(a) 1/2
(b) 1/√2
(c) 1/3
(d) 1/√3
89. The area bounded by the loop of the curve 4y2 = x2(4 – x2) is
(a) 7/3 square units
(b) 8/3 square units
(c) 11/3 square units
(d) 16/3 square units
90. The integrating factor of the linear differential equation ![]() is
is
(a) 
(b) 
(c) 
(d) 
91. A particle moves in a straight line with velocity given by ![]() (x being the distance described). The time taken by the particle to describe 99 metres is
(x being the distance described). The time taken by the particle to describe 99 metres is
(a) log10 e
(b) 2loge 10
(c) 2log10 e
(d) ![]()
92. The number of integer values of m, for which the x-co-ordinate of the point of intersection of the lines 3x + 4y = 9 and y = mx + 1 is also an integer is
(a) 2
(b) 0
(c) 4
(d) 1
93. Equations of the bisectors of the lines 3x− 4y + 7=0 and 12x + 5y −2 = 0 are given by
(a) 21x + 77y – 101 = 0, 11x – 3y + 9 = 0
(b) 11x – 6y + 111 = 0, 22x – 13y + 104 = 0
(c) 15x – 9y + 67 = 0, 15x + 4y + 33 = 0
(d) 20x + 72y – 109 = 0, x + 5y = 2
94. The circles x2 + y2 – 4x – 6y – 12 = 0 and x2 + y2 + 6x – 8y + 21 = 0
(a) intersect at two points
(b) touches each other externally
(c) touches each other internally
(d) neither touches nor intersects
95. The length of the intercept made by the line y = 2x + 1 on the circle x2 + y2 = 2 is
(a) 6/√5
(b) 6√5
(c) 6√2
(d) 6/√2
96. The orthocenter of the triangle formed by the points t1, t2, t3 on the parabola y2 = 4ax is
(a) vertex
(b) focus
(c) origin
(d) (1, 0)
97. The equation of tangent to the ellipse x2 + 4y2 = 25 at the point whose ordinate is 2, is
(a) x + 2y or 2x – y = 5
(b) 3x + 8y = 25 or 8y – 3x = 25
(c) 3x + 2y = 15 or 3y – 2x = 15
(d) x + y = 1 or 2x – y = 5
98. The vector that should be added to ![]() so that resultant is the unit vector
so that resultant is the unit vector ![]() is
is
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
99. If the vectors ![]() are non collinear and the vectors
are non collinear and the vectors ![]() and
and ![]() are collinear then x is equal to
are collinear then x is equal to
(a) x = −1/3
(b) x = 2/3
(c) x = 0
(d) x = 1/3
100. In an experiment with 15 observations on x the following results were available ![]() One observation 20 found to be wrong was replaced by the correct value 30. Then, the corrected variance is
One observation 20 found to be wrong was replaced by the correct value 30. Then, the corrected variance is
(a) 188.66
(b) 177.33
(c) 8.33
(d) 78.00
101. Let u1 and u2 be two wins such that u1 contains 3 white and 2 red balls and u2 contains only one white ball. A fair coin is tossed. If head appear then one ball is drawn at random from u1 and put into u2. However if tail appears then 2 balls are drawn at random from u1 and put into u2. Now one ball is drawn at random from u2. Then the probability of the drawn ball from u2 being white is
(a) 13/30
(b) 23/30
(c) 19/30
(d) 11/30
102. Let ![]()
![]() then tan 2α is
then tan 2α is
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
103. If 1 + sin x + sin2x + sin3 x + … ∞ = 4 + 2√3, 0 < x < π then x is equal to,
(a) π /6
(b) π/4
(c) π/3 or π/6
(d) π/3 or 2π/3
104. If f(x) = xn, then the value of ![]() where fr(x) denotes the rth order derivative of f(x) with respect to x is
where fr(x) denotes the rth order derivative of f(x) with respect to x is
(a) n
(b) 2n
(c) 2n – 1
(d) n2n – 1
105. The value of  is equal to
is equal to
(a) 5(2n – 9)
(b) 10n
(c) 9(n – 4)
(d) 10(n – 1)
Part 4 – Biology
71. The Human Genome Project officially began in
(a) 1988
(b) 1990
(c) 1992
(d) 1998
72. What is an argument in favor of using embryonic stem cells over adult stem cells?
(a) Embryonic stem cells are never really living.
(b) Embryonic stem cells can differentiate into many more types of cells.
(c) Adult stem cells cannot be cultured.
(d) Adult stem cells reproduce much faster than embryonic stem cells.
73. The analysis and storage of the massive amount of data generated from sequence maps has led to the growth of what new disciplines?
(a) Bioinformatics and medical microbiology
(b) Genomics and genetic engineering
(c) Genomics and bioinformatics
(d) Proteomics and environmental microbiology
74. Which technique is not used in the transfer of gene into fertilized egg or embryo?
(a) Fusion using polyethylene glycol
(b) Hypotonic lysis
(c) Microinjection
(d) Polymerization
75. The DNA molecule to which the gene of interest is integrated for cloning is called
(a) Carrier
(b) Vector
(c) Transformant
(d) cDNA
76. Totally unrelated plants are brought together in a single group and those that are closely related are placed in widely separated groups in the system of classification given by ___________.
(a) Bentahm and Hooker
(b) Carolus Linnaeus
(c) Engler and Prantl
(d) Charles Darwin
77. In Pavonia odorata the inflorescence is ___________.
(a) terminal head
(b) axillary cyathium
(c) terminal or axillary cyme
(d) axillary cyme
78. Leaves are simple and whorled in __________.
(a) Ixora
(b) Galium
(c) Gardeni
(d) Rubia
79. Morphologically, a _____________is a group of cells, which are similar in origin, form and function.
(a) tissue
(b) tissue system
(c) organ
(d) organ system
80. Differentiation is change of tissues from___________.
(a) meristematic to permanent
(b) simple to complex
(c) complex to simple
(d) permanent to meristematic
81. Procambium gives rise to _____________
(a) primary vascular tissues
(b) epidermis
(c) cortex
(d) pith
82. Cellulose is a polymer of D-glucose units joined by
(a) α1 – 4 linkage
(b) α1 – 6 linkage
(c) β1 – 4 linkage
(d) β1 – 6 linkage
83. The most accepted theory of origin of life is
(a) Special creation theory
(b) Theory of abiogenesis
(c) Oparin haldane theory
(d) Theory of spontaneous generation
84. One of the following structure of Cockroach is responsible for water conservation.
(a) Spiracles
(b) Hepatic caeca
(c) Malpighian tubules
(d) short midgut
85. A DNA strand with the sequence AACGTAACG is transcribed.What is the sequence of the mRNA molecule synthesized?
(a) AACGTAACG
(b) UUGCAUUGC
(c) AACGUAACG
(d) TTGCATTGC
86. Which of the following is not a component of cell membrane?
(a) Lipid
(b) Nucleic acids
(c) Carbohydrate
(d) Proteins
87. Golden rice is a transgenic crop of the future with the following improved trait
(a) High lysine content
(b) Insect resistance
(c) High protein content
(d) High Vitamin-A content
88. When the genotype of an organism is improved by the addition of foreign gene, the process is called
(a) Tissue culture
(b) Genetic diversity
(c) Genetic engineering
(d) Plastic surgery
89. An example for transferase is
(a) transminase
(b) pyruvic carboxylase
(c) histidine decarboxylase
(d) G-3-P dehydrogenase
90. The Non-cyclic photophosphorylation is associated with
(a) PS-I
(b) PS-II
(c) PS-I & II
(d) PIS.
91. In C3 plants, photosynthesis occurs in
(a) Mesophyll cells
(b) Bundle sheath cells
(c) Both mesophyll and bundle sheath cells
(d) Cortex cells
92. Which of the following is an ephyphitic plants?
(a) Rice
(b) Vanda
(c) Cuscuta
(d) Viscum
93. Which of the following is the common respiratory substrate?
(a) Proteins
(b) Lipids
(c) Carbohydrates
(d) Vitamins
94. In order to obtain disease-free plants through tissue culture techniques, the best method is
(a) Protoplast culture
(b) Anther culture
(c) Embryo rescue
(d) Meristem culture
95. Consider the following statements (A-D) about organic farming
A. Utilizes genetically modified crops like Bt cotton
B. Uses only naturally produced inputs like compost
C. Does not use pesticides and urea
D. Produces vegetables rich in vitamins and minerals
Which of the above statements are correct?
(a) (B) and (C) only
(b) (A) and (B) only
(c) (B), (C) and (D)
(d) (C) and (D) only
96. Which one of the following is linked to the discovery of Bordeaux mixture as a popular fungicide?
(a) Black rust of wheat
(b) Bacterial leaf blight of rice
(c) Downy mildew of grapes
(d) Loose smut of wheat
97. The method of producing thousands of plants through tissue culture is called
(a) Micropropagation
(b) Somatic hybridization
(c) Biofortification
(d) Biomagnification
98. Saffron is produced from
(a) Roots of Indigofera
(b) Petals of Rosa
(c) Stamens of Hibiscus
(d) Style and stigma of Crocus
99. Joint dislocation caused by poliomyelitis is
(a) Congenital
(b) Traumatic
(c) Pathological
(d) Paralytic
100. Which of the following is not a regulatory function of lungs?
(a) Water balance
(b) Acid – base
(c) Body temperature
(d) Heart rate
101. The inhibitory process of respiratory centre in brain that regulates the extent of inspiration is known as
(a) Pavlov reflex
(b) Spinal reflex
(c) Neuro – endocrine reflex
(d) Herring – Breuer reflex
102. Atrophy of cerebral cortex leads to
(a) Stroke
(b) Meningitis
(c) Alzheimers
(d) Parkinsons
103. Synthesis and secretion of testosterone is regulated by
(a) ACTH
(b) FSH
(c) LH
(d) LTH
104. Largest organ in human system is
(a) Liver
(b) Brain
(c) Lungs
(d) Skin
105. Which of the following hormone has metabolic effects opposite to that of insulin?
(a) Calcitonin
(b) Testosterone
(c) Mineralocorticoid
(d) Glucocorticoid
106. Which of the following does not belong to lower respiratory tract?
(a) Larynx
(b) Trachea
(c) Bronchi
(d) Lungs
107. Testosterone is produced by ___________ cells in testes.
(a) Interstitial
(b) Sertoli
(c) Nurse
(d) vas deferens
108. A protozoan is generally called as
(a) motile procaryotic unicellular protest
(b) motile eucaryotic unicellular protest
(c) motile eucaryotic unicellular photosynthetic protist
(d) motile eucaryotic multicellular protest.
109. Which is the most widely used physical method for microbial control?
(a) Filtration
(b) Ionization
(c) Radiation
(d) Sterilization
110. A genetically engineered microorganism used successfully in bioremediation of oil spills is a species of
(a) Trichoderma
(b) Bacillus
(c) Xanthomonas
(d) Pseduomonas
111. The following organisms have been proposed as a best source of single cell protein.
(a) Cyanobacteria
(b) Aspergillus
(c) Bacillus
(d) Pseudomonas
112. Which of the following does not protect body surfaces?
(a) Skin
(b) Mucus
(c) Gut microflora
(d) Salivary amylase
113. The basic Ig unit is composed of :
(a) Two identical heavy and two identical light chains
(b) Two identical heavy and two different light chains
(c) Two different heavy and two identical light chains
(d) Two different heavy and two different light chains
114. Which of the following is NOT causing the net CO2 in the atmosphere to increase?
(a) photosynthesis
(b) burning gasoline
(c) deforestation
(d) burning coal
115. Endemic species are
(a) rare species
(b) species localized in a specific region
(c) cosmopolitan in distribution
(d) critically endangered species
116. The law which ensures environmental stability and maintenance of ecological balance is
(a) Forest Act 1927
(b) Wildlife Act1972
(c) Wild life protection Act 1991
(d) National forest policy 1988
117. Animal pharming can be defined as
(a) Growing animals for farming
(b) Generating transgenic animals for farming
(c) Programming animals to produce novel products
(d) Treatment for farming animals
118. The accuracy of estimating breeding value of a sire increased by ———
(a) Decreasing the number of sires under test
(b) Decreasing the number of progeny of sire
(c) Increasing the number of progeny of sire
(d) Remains constant number of progeny of sire
119. The raw material for evolution is
(a) Selection
(b) Genetic drift
(c) Mutation
(d) migration
120. One of the important consequences of geographical isolation is
(a) Preventing Speciation
(b) Random creation of new species
(c) No change in the isolated fauna
(d) Speciation through reproductive isolation
Latest Govt Job & Exam Updates: