AIIMS MBBS Entrance Exam – 2008
Physics
1. A string is stretched between fixed points separated by 75.0 cm. It is observed to have resonant frequencies of 420 Hz and 315 Hz. There are no other resonant frequencies between these two. Then, the lowest resonant frequency for this string is
(a) 105 Hz
(b) 1.05 Hz
(c) 1050 Hz
(d) 10.5 Hz
2. If the terminal speed of a sphere of gold (density = 19.5 kg/m3) is 0.2 m/s in viscous liquid (density = 1.5 kg/m3), find the terminal speed of a sphere of silver (density = 10.5 kg/m3) of the same size in the same liquid.
(a) 0.4 m/s
(b) 0.133 m/s
(c) 0.1 m/s
(d) 0.2 m/s
3. A coin is placed on a horizontal platform which undergoes vertical simple harmonic motion of angular frequency ω. The amplitude of oscillation is gradually increased. The coin will leave contact with the platform for the first time
(a) at the mean position of the platform
(b) for an amplitude of g/ω2
(c) for an amplitude of g2/ω2
(d) at the highest position of the platform
4. Four point masses, each of value m, are placed at the corners of square ABCD is side l. The moment of inertia of this system about an axis passing through A and parallel to BD is
(a) 2 ml2
(b) √3 ml2
(c) 3 ml2
(d) ml2
5. Two rigid boxes containing different ideal gases are placed on table. Box A contains one m ole of nitrogen at temperature T0, while box B contains one mole of helium at temperature (7/3)T0. The boxes are then put into thermal contact with each other, and heat flows between them until the gases reach a common final temperature (Ignore the heat capacity of boxes). Then, the final temperature of the gases Tf, in terms of T0 is
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
6. Three point charges +q, −2q and +q are placed at points (x = 0, y = a, z = 0), (x = 0, y = 0, z = 0) and (x = a, y = 0, z = 0), respectively. The magnitude and direction of the electric dipole moment vector of this charge assembly are
(a) √2qa along +y direction
(b) √2 aq along the line joining points (x = 0, y = 0, z = 0) and (x = a, y = a, z = 0)
(c) qa along the line joining points (x = 0, y = 0, z = 0) and (x = a, y = a, z = 0)
(d) √2 qa along +x direction
7. A long straight wire of radius a carries a steady current I. The current is uniformly distributed across its cross-section. The ratio of the magnetic field at a/2 and 2a is
(a) 1/4
(b) 4
(c) 1
(d) 1/2
8. For the given uniform square lamina ABCD, whose centre is O
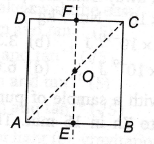
(a) √2IAC = IEF
(b) IAD = 3IEF
(c) IAC = IEF
(d) IAC = √2IEF
9. If in a p-n junction diode, a square input signal of 10 V is applied as shown
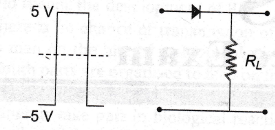
Then the output signal across RL will be
(a) 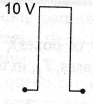
(b) 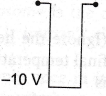
(c) 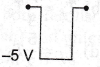
(d) 
10. A wheel has angular acceleration of 3.0 rad/s2 and an initial angular speed of 2.00 rad/s. In a time of 2 s it has rotated through an angle (in radian) of
(a) 6
(b) 10
(c) 12
(d) 4
11. A hollow cylinder h as a charge q C within it. If ϕ is the electric flux in unit of voltmeter associated with the curved surface B, the flux linked with the plane surface A in unit of voltmeter will be
(a) 
(b) ![]()
(c) ![]()
(d) ![]()
12. A particle of mass 10 g is kept on the surface of a uniform sphere of mass 100 kg and radius 10 cm. Find the work to be done against the gravitational force between them, to take the particle far away from the sphere (you may take G = 6.67 × 10−11 Nm2/kg2)
(a) 13.34 × 10−10 J
(b) 3.33 × 10−10 J
(c) 6.67 × 10−9 J
(d) 6.67 × 10−10 J
13. Starting with a sample of pure 66 Cu, 7/8 of it decays into Zn in 15 min. The corresponding half-life is
(a) 10 min
(b) 15 min
(c) 5 min
(d) ![]()
14. A smooth block is released at rest on a 45° incline and then slides a distance d. .The time taken to slide is n times as much to slide on rough incline than on a smooth incline. The coefficient of friction is
(a) ![]()
(b) 
(c) ![]()
(d) ![]()
15. A projectile can have the same range R for two angles of projection. If t1 and t2 be the times of flights in the two cases, then the product of the two times of flights is proportional to
(a) R2
(b) 1/R2
(c) 1/R
(d) R
16. A parachutist after bailing out falls 50 m without friction. When parachute opens, it decelerates at 2 m/s2. He reaches the ground with a speed of 3 m/s. At what height, did he bail out?
(a) 91 m
(b) 182 m
(c) 293 m
(d) 111 m
17. A car, starting from rest, accelerates at the rate f through a distance S, then continues at constant speed for time t and then decelerates as the rate f/2 to come to rest. If the total distance travelled is 15 S, then
(a) S = ft
(b) ![]()
(c) ![]()
(d) ![]()
18. In a mass spectrometer used for measuring the masses of ions, the ions are initially accelerated by an electric potential V and then made to describe semicircular paths of radius R using a magnetic field B. if V and B are kept constant, the ratio 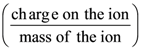 will be proportional to
will be proportional to
(a) 1/R
(b) 1/R2
(c) R2
(d) R
19. Two sources of equal emf are connected to an external resistance R. The internal resistances of the two sources are R1 and R2 (R2 > R1). If the potential difference across the source having internal resistance R2, is zero, then
(a) 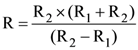
(b) R = R2 – R1
(c) 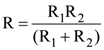
(d) 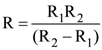
20. Two point white dots are 1 mm apart on a black paper. They are viewed by eye of pupil diameter 3 mm. Approximately, what is the maximum distance at which these dots can be resolved by the eye? [Take wavelength of light = 550 mm]
(a) 5 m
(b) 1 m
(c) 6 m
(d) 3 m
21. The function sin2 (ωt) represents
(a) a periodic, but not simple harmonic motion with a period 2π/ω
(b) a periodic, but not simple harmonic motion with a period π/ω
(c) a simple harmonic motion with a period 2π/ω
(d) a simple harmonic motion with a period π/ω
22. A thin glass (refractive index 1.5) lens has optical power of −5 D in air. Its optical power in a liquid medium with refractive index 1.6 will be
(a) 1 D
(b) −1 D
(c) 25 D
(d) −25 D
23. A fish looking up through the water sees the outside world, contained in a circular horizon. If the refractive index of water is 4/3 and the fish is 12 cm below the water surface, the radius of this circle in cm is
(a) 36√7
(b) 36/√7
(c) 36√5
(d) 4√5
24. The temperature-entropy diagram of a reversible engine cycle is given in the figure. Its efficiency is
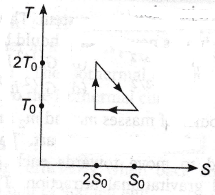
(a) 1/2
(b) 1/4
(c) 1/3
(d) 2/3
25. A vertical spring with force constant k is fixed on a table. A ball of mass m at a height h above the free upper end of the spring falls vertically on the spring, so that the spring is compressed by a distance d. The net work done in the process is
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
26. Two concentric coils each of radius equal to 2π cm are placed at right angle to each other. 3 A and 4 A are the currents flowing in each coil respectively. The magnetic induction in Wb/m2 at the centre of the coils will be
(μ0 = 4π × 10−7 Wb/m)
(a) 12 × 10−5
(b) 10−5
(c) 5 × 10−5
(d) 7 × 10−5
27. The input resistance of a common emitter transistor amplifier, if the output resistance is 500 kΩ, the current gain α = 0.98 and power gain is 6.0625 × 106, is
(a) 198 Ω
(b) 300 Ω
(c) 100 Ω
(d) 400 Ω
28. A car travels 6 km toward north at an angle of 45° to the east and then travels distance of 4 km towards north at an angle 135° to east. How far is the point from the starting point? What angle does the straight line joining its initial and final position makes with the east?
(a) √50 km and tan−1 (5)
(b) 10 km and tan−1 (√5)
(c) √52 km and tan−1 (5)
(d) √52 km and tan−1 (√5)
29. The speed of light (c), gravitational constant (G) and Planck’s constant (h) are taken as fundamental units in a system. The dimensions of time in this new system should be
(a) G1/2 h1/2 c−5/2
(b) G−1/2 h1/2 c1/2
(c) G1/2 h1/2 c−3/2
(d) G1/2 h1/2 c1/2
30. Two bodies of masses m1 and m2 are initially at rest at infinite distance apart. They are then allowed to move towards each other under mutual gravitational attraction. Their relative velocity of approach at a separation distance r between them is
(a) 
(b) 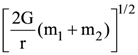
(c) 
(d) 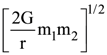
31. The adjacent graph shows the extension (∆l) of a wire of length 1 m suspended from the top of a root at one end with a load W connected to the other end. If the cross-sectional area of the wire is 10−6 m2, calculate the Young’s modulus of the material of the wire.

(a) 2 × 1011 N/m2
(b) 2 × 10−11 N/m2
(c) 3 × 10−12 N/m2
(d) 2 × 10−13 N/m2
32. Water is filled in a cylindrical container to a height of 3 m. The ratio of the cross-sectional area of the orifice and the beaker is 0.1. The square of the speed of the liquid coming out from t he orifice is (g = 10 m/s2)
(a) 50 m2/s2
(b) 50.5 m2/s2
(c) 51 m2/s2
(d) 52 m2/s2
33. A particle of mass m is executing oscillations about the origin on the x-axis. Its potential energy is U(x) = k[x]3, where k is a positive constant. If the amplitude of oscillation is a, then its time period T is
(a) proportional to 1/√a
(b) independent of a
(c) proportional to √a
(d) proportional to a3/2
34. A person speaking normally produces a sound intensity of 40 dB at a distance of 1 m. If the threshold intensity for reasonable audibility is 20 dB, the maximum distance at which he can be heard clearly is
(a) 4 m
(b) 5 m
(c) 10 m
(d) 20 m
35. Charge q is uniformly distributed over a thin half ring of radius R. The electric field at the centre of the ring of
(a) 
(b) 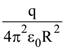
(c) 
(d) 
36. In the figure shown, the capacity of t he condenser C is 2μ The current in 2 Ω resistor is
(a) 9 A
(b) 0.9 A
(c) ![]()
(d) ![]()
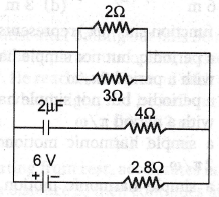
37. When the key K is pressed at t = 0, which of the following statements about the current I in the resistor AB of the given circuit is true?
(a) I = 2 mA at all t
(b) I oscillates between 1 mA and 2 mA
(c) I = 1 mA at all t
(d) At t = 0, I = 2 mA and with time it goes to 1 mA
38. An AC source of angular frequency ω is fed across a resistor R and a capacitor C in series. The current registered is I. If now the frequency of source is changed to ω/3 (but maintaining the same voltage), the current in then circuit is found to be halved. Calculated the ratio of reactance to resistance at the original frequency ω
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
39. A radioactive material decays by simultaneous emission of two particles with respective half lives 1620 yr and 810 yr. The time (in years) after which one-fourth of the material remains is
(a) 1080
(b) 2430
(c) 3240
(d) 4860
40. In a photoemissive cell with executing wavelength λ, the fastest electron has speed v. If the exciting wavelength is changed to 3λ/4, the speed of the fastest emitted electron will be
(a) v(3/4)1/2
(b) v(4/3)1/2
(c) less than v(4/3)1/2
(d) greater than v(4/3)1/2
Directions for Q. 41 to Q. 60 : In each of the following questions a statement of Assertion is given followed by a corresponding statement of Reason just below it. Of the statements mark the correct answer as
(a) If both Assertion and Reason are true and the Reason is the correct explanation of the Assertion.
(b) If both Assertion and Reason are true but the Reason is not the correct explanation of the Assertion.
(c) If Assertion is true but Reason is false.
(d) If both Assertion and Reason are false.
41. Assertion : The error in the measurement of radius of the sphere is 0.3%. The permissible error in its surface area is 0.6%.
Reason : The permissible error is calculated by the formula ![]()
42. Assertion : The isothermal curves intersect each other at a certain point.
Reason : The isothermal changes takes place rapidly, so the isothermal curves have very little slope.
43. Assertion : An electric motor will have maximum efficiency when back emf becomes equal to half of applied emf.
Reason : Efficiency of electric motor depends only on magnitude of back emf.
44. Assertion : In electric circuits, wires carrying currents in opposite directions are often twisted together.
Reason : If the wires are not twisted together, the combination of the wires forms a current loop, The magnetic field generated by the loop might affect adjacent circuits or components.
45. Assertion : Balmer series lies in the visible region of electromagnetic spectrum.
Reason :  where = n = 3, 4, 5.
where = n = 3, 4, 5.
46. Assertion : The velocity of a body at the bottom of an inclined plane of given height is more when it slides down the plane, compared to, when it rolling down the same plane.
Reason : In rolling down a body acquires both, kinetic energy of transiation and rotation.
47. Assertion : A body of mass 1 kg is making 1 rps in a circle of radius 1 m. Centrifugal force acting on it is 4π2
Reason : Centrifugal force is given by ![]()
48. Assertion : The change in air pressure effects the speed of sound.
Reason : The speed of sound in gases is proportional to the square of pressure.
49. Assertion : A parallel plate capacitor is connected across battery through a key. A dielectric slab of dielectric constant K is introduced between the plates. The energy which is stored becomes K times.
Reason : The surface density o f charge on the plate remains constant or unchanged.
50. Assertion : If a compass needle be kept a magnetic north pole of the earth, the compass needle may stay in any direction.
Reason : Dip needle will stay vertical at the north pole of earth.
51. Assertion : Displacement current goes through the gap between the plates of a capacitor when the charge of the capacitor does not change.
Reason : The displacement current arises in the region in which the electric field and hence the electric flux does not change with time.
52. Assertion : An electric bulb becomes dim, when an electric heater in parallel circuit is switched on.
Reason : Dimness decreases after sometime.
53. Assertion : The magnetic field produced by a current carrying solenoid is independent of its length and cross-sectional area.
Reason : The magnetic field inside the solenoid is uniform.
54. Assertion : Angle o f repose is equal to the angle of limiting friction.
Reason : When the body is just at the point of motion, the force of friction in this stage is called limiting friction.
55. Assertion : A quick collision between two bodies is more violent than a slow collision; even when the initial and final velocities are identical.
Reason : The momentum is greater in first case.
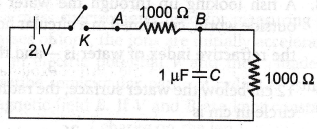
56. Assertion : The value of current through p-n junction in the given figure will be 10 mA.
Reason : In the above figure, p-side is at higher potential than n-side.
57. Assertion : A concave mirror and convex lens both have the same focal length in air. When they are submerged in water, they will have same focal length.
Reason : The refractive index of water is smaller than the refractive index of air.
58. Assertion : A bubble comes from the bottom of a lake to the top.
Reason : Its radius increases.
59. Assertion : In Young’s double slit experiment the two slits are at distance d apart. Interference pattern is observed on a screen at distance D from the slits. At a point on the screen when it is directly opposite to one of the slits, a dark fringe is observed. Then, the wavelength of wave is proportional to square of distance of two slits.
Reason : For a dark fringe intensity is zero.
60. Assertion : Mean free path of a gas molecules varies inversely ad density of the gas.
Reason : Mean free path varies inversely as pressure of the gas.
AIIMS MBBS Entrance Exam – 2008
Chemistry
1. C8H6 that can from cis-trans geometrical isomers and also has a chiral centre, is
(a) 
(b) 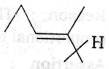
(c) Both of these
(d) None of these
2. Select correct statement (s).
(a) Cyanamide ion (CN22−) is isoelectronic with CO2 and has the same linear structure
(b) Mg2C3 reacts with water to form propyne
(c) CaC2 has NaCl type lattice
(d) All of the above
3. Perdisulphuric acid has the following bond
(a) O ← O = O
(b) ← O = O →
(c) > O → O <
(d) −O−O−
4. The energy equivalent of 2.0 mg mass defect is
(a) 1.8 × e4 erg
(b) 9 × 10−19 erg
(c) 1.5 × 1020 erg
(d) 1.8 × 1018 erg
5. Which of the following compounds is coloured?
(a) TiCl3
(b) FeCl3
(c) CoCl2
(d) All of these
6. ![]()
The compound X is
(a) SnCl2/HCl/H2O, boil
(b) H2/Pd – BasO4
(c) LiAlH4/ether
(d) NaBH4/ether/H3O+
7. The compound which gives an oily nitrosoamine on reaction with nitrous acid at low temperature, is
(a) CH3NH2
(b) (CH3)2CHNH2
(c) CH3 – NH – CH3
(d) (CH3)3N
8. In which of the following reactions the product obtained is t-butyl methyl ether?
(a) ![]()
(b) 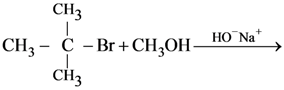
(c) 
(d) 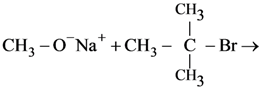
9. Non-oxide ceramics can be
(a) B4C
(b) SiC
(c) Si3N4
(d) All of these
10. What is the pH value of M H2SO4?
(a) 0
(b) −0.213
(c) −2
(d) −0.03010
11. For a first order reaction, to obtain a positive slope, we need to plot {where [A] is the concentration of reactant A}
(a) −log10[A] vs t
(b) −loge[A] vs t
(c) log10[A] vs log t
(d) [A] vs t
12. 01 M solution of KCl and BaCl2 are prepared in water. The freezing points of KCl is found to be −2°C. What is the freezing point of BaCl2 to be completely ionized?
(a) −3°C
(b) +3°C
(c) −2°C
(d) −4°C
13. Sulphur reacts with chlorine in 1 : 2 ratio and forms X. Hydrolysis of X gives a sulphur compound Y. What is the structure and hybridization of anion of Y?
(a) tetrahedral, sp3
(b) linear, sp
(c) pyramidal, sp3
(d) trigonal planar, sp2
14. Which of the following is a primary halide?
(a) Iso-propyl iodide
(b) Secondary butyl iodide
(c) Tertiary butyl bromide
(d) Neo-hexyl chloride
15. 6C12 and 1T3 are formed in nature due to the nuclear reaction of neutron with
(a) 7N14
(b) 6C13
(c) 2He4
(d) 3Li6
16. The correct structure of 4-bromo-3-methyl but-1-ene is
(a) Br – CH = C(CH3)2
(b) CH2 = CH – CH(CH3) – CH2Br
(c) CH2 = C(CH3)CH2CH2Br
(d) CH3 – C(CH3) = CHCH2 – Br
17. The chemical formula of ‘tear gas’ is
(a) COCl2
(b) CO2
(c) Cl2
(d) CCl3NO2
18. During electrolysis of water the volume of O2 liberated is 2.24 dm3. The volume of hydrogen liberated, under same conditions will be
(a) 2.24 dm3
(b) 1.12 dm3
(c) 4.48 dm3
(d) 0.56 dm3
19. 3-hyroxy butanal is formed when X reacts with Y in dilute Z solution. What are X, Y and Z ?
(a) X – CH3CHO ; Y – (CH3)2CO ; Z – NaOH
(b) X – CH3CHO ; Y – CH3CHO ; Z – NaCl
(c) X – (CH3)2CO ; Y – (CH3)2CO ; Z – HCl
(d) X – CH3CHO ; Y – CH3CHO ; Z – NaOH
20. In which of the following reactions, the concentration of the product is higher than the concentration of reactant at equilibrium?
(K = equilibrium constant)
(a) A ⇌ B; K = 0.001
(b) M ⇌ N; K = 10
(c) X ⇌ Y; K = 0.005
(d) R ⇌ P; K = 0.01
21. The statement which is not correct, is
(a) chlorophyll is responsible for the synthesis of carbohydrates in plants
(b) the compound formed by the addition of oxygen to haemoglobin is called oxyhaemoglobin
(c) acetyl salicyclic acid is known as aspirin
(d) the metal ion present in vitamin B12 is Mg2+
22. Acid hydrolysis of which of the following compounds yields two different organic compounds?
(a) CH3COOH
(b) CH3CONH2
(c) CH3COOC2H5
(d) (CH3CO)2O
23. The emf of Daniell cell at 298 K is E1 Zn | ZnSO4 (0.01 m)| | CuSO4 (1.0 M)| Cu
When the concentration of ZnSO4 is 1.0 M and that of CuSO4 is 0.01 M, the emf changed to E2. What is the relation between E1 and E2?
(a) E1 = E2
(b) E2 = 0 ≠ E2
(c) E1 > E2
(d) E1 < E2
24. Which of the following is a lyophobic colloidal solution?
(a) Aqueous starch solution
(b) Aqueous protein solution
(c) Gold sol
(d) Polymer solvent in some organic solvents
25. The uncertainties in the velocities of two particles, A and B are 0.05 and 0.02 ms−1, respectively. The mass of B is five times to that of the mass of A. What is the ratio of uncertainties 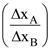 in their positions?
in their positions?
(a) 2
(b) 0.25
(c) 4
(d) 1
26. The volume-temperature graphs of a given mass of an ideal gas at constant pressure are shown below.
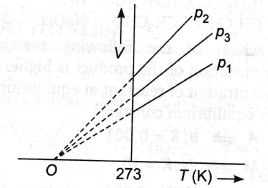
What is the correct order of pressures?
(a) p1 > p3 > p2
(b) p1 > p2 > p3
(c) p2 > p3 > p1
(d) p2 > p1 > p3
27. For a reaction to be spontaneous at all temperatures
(a) ∆G –ve, ∆H +ve and ∆S +ve
(b) ∆G +ve, ∆H –ve and ∆S +ve
(c) ∆G –ve, ∆H –ve, and ∆S –ve,
(d) ∆G –ve, ∆H –ve, and ∆G +ve
28. The oxidation states of iodine in HIO4, H3IO5 and H5IO6 are, respectively
(a) +1, +3, +7
(b) +7, +7, +3
(c) +7, +7, +7
(d) +7, +5, +3
29. What will be the heat of formation of methane, if the heat of combustion of carbon is ‘−x’ kJ, heat of formation of water is ‘−y’ kJ and heat of combustion of methane is ‘z’ kJ?
(a) (−x – y + z) kJ
(b) (−z – x + 2y) kJ
(c) (−x – 2y – z) kJ
(d) (−x – 2y + z) kJ
30. Which of the following is a polymer containing nitrogen?
(a) Polyvinyl chloride
(b) Bakelite
(c) Nylon
(d) Terylene
31. The beta and alpha glucose have different specific rotations. When either is dissolved in water, their rotation changes until the same fixed value results. This is called
(a) epimerisation
(b) racemisation
(c) anomerisation
(d) mutarotation
32. An organic compound X on treatment with pyridinium chloro chromate in dichloromethane gives compound Y. Compound Y reacts with I2 and alkali to form triiodomethane. The compound ‘X’ is
(a) C2H5OH
(b) CH3CHO
(c) CH3COCH3
(d) CH3COOH
33. [Fe(NO2)3Cl3] and [Fe(O – NO)3Cl3] shows
(a) linkage isomerism
(b) geometrical isomerism
(c) optical isomerism
(d) None of the above
34. Maximum enol content is in
(a) 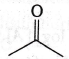
(b) 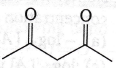
(c) 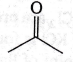
(d) 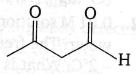
35. T50 of first-order reaction is 10 min. Starting with 10 mol L−1, rate after 20 min is
(a) 0.0693 mol L−1 min−1
(b) 0.693 × 2.5 mol L−1 min−1
(c) 0.0693 × 5 mol L−2 min−1
(d) 0.0693 mol L−1 min−1
36. On adding 0.1 M solution each of [Ag+], [Ba2+], [Ca2+] in a Na2SO4 solution, species first precipitated is
[Ksp BaSO4 = 10−11, Ksp CaSO4 = 10−6, Ksp Ag2SO4 = 10−5]
(a) Ag2SO4
(b) BaSO4
(c) CaSO4
(d) All of these
37. Spin isomerism is shown by
(a) dichloro benzene
(b) hydrogen
(c) dibasic acid
(d) n-butane
38. 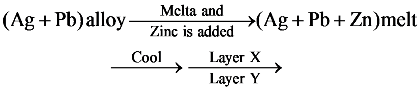
Select correct statement based on above scheme.
(a) Layer X contains Zn and Ag
(b) Layer Y contains Pb and Ag but amount of silver in this layer is smaller than in layer X
(c) X and Y are immiscible layer
(d) All are correct statements
39. S2− and SO32− can be distinguished by using
(a) (CH3COO)2Pb
(b) Na2[Fe(CN)5NO]
(c) both (a) and (b)
(d) None of these
40. Which of the following molecules can act as an oxidizing as well as a reducing agent?
(a) H2S
(b) SO3
(c) H2O2
(d) F2
Directions for Q. 41 to Q. 60 : In each of the following questions a statement of Assertion is given followed by a corresponding statement of Reason just below it. Of the statements mark the correct answer as
(a) If both Assertion and Reason are true and the Reason is the correct explanation of the Assertion.
(b) If both Assertion and Reason are true but the Reason is not the correct explanation of the Assertion.
(c) If Assertion is true but Reason is false.
(d) If both Assertion and Reason are false.
41. Assertion : A spectral line will be observed for a 2px-2py
Reason : The energy is released in the form of wave of light when electron drops from 2px to 2py orbital
42. Assertion : Colloidal solutions are stable but colloidal particles do not settle down.
Reason : Brownian movement counters the force o f gravity actively on colloidal particles.
43. Assertion : The S–S–S bond angle in S8 molecule is 105°.
Reason : S8 has V-shape.
44. Assertion : [Al(H2O)6]3+ is a stronger acid than [Mg(H2O)6]2+.
Reason : Size of [Al(H2O)6]3+ is smaller than [Mg(H2O)6]2+ and possesses more effective nuclear charge.
45. Assertion : Oils are purified by steam distillation.
Reason : The compounds which decompose at their boiling points can purified by steam distillation.
46. Assertion : Friedel-Craft’s reaction is used to introduce an alkyl or acyl group in benzene nucleus.
Reason : Benzene is a solvent for the Friedel-Craft’s alkylation of bromobenzene.
47. Assertion : Disruption of the natural structure of a protein is called denaturation.
Reason : The change in colour and appearance of egg during cooking is due to denaturation.
48. Assertion : Equivalent weight of a base
![]()
Reason : Acidity is the number of replaceable hydrogen atoms in one molecule of the base.
49. Assertion : Fluorine molecule has bond order one.
Reason : The number of electrons in the antibonding molecular orbitals is two less than that in bonding molecular orbitals.
50. Assertion : For a reaction
2NH3(g) → N2(g) + 3H2(g); ∆H > ∆E
Reason : Enthalpy change in always greater than internal energy change.
51. Assertion : One molal aqueous solution of glucose contains 180 g of glucose in 1 kg water.
Reason : Solution containing one mole of solute in 100 g of solvent is called one molar solution.
52. Assertion : PbCl2 is more stable than PbCl4.
Reason : PbCl4 is powerful oxidizing agent.
53. Assertion : The ease of dehydration of the following alcohols is
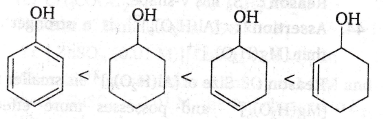
Reason : Alcohols leading to conjugated alkenes are dehydrated to a greater extent.
54. Assertion : No compound has both Schottky and Frenkel defects.
Reason : Both defects change the density of the solid.
55. Assertion : The order of a reaction can have fractional value.
Reason : The order of a reaction cannot be written from balanced equation o f a reaction.
56. Assertion : For the reaction
N2(g) + 3H2(g) ⇌ 2NH3(g)
Unit of Kc = L2 mol−2
Reason : For the reaction
N2(g) + 3H2(g) ⇌ 2NH3(g)
Equilibrium constant, 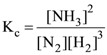
57. Assertion : Tropylium cation is aromatic in nature.
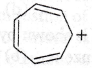
Reason : The only property that determines its aromatic behaviour is its planar structure.
58. Assertion : Nitrobenzene is used as a solvent in Friedel-Craft’s reaction.
Reason : Fusion of nitrobenzene with solid KOH gives a low yield of a mixture of o- and p-nitrop phenols.
59. Assertion : Boiling points of cis-isomers are higher than trans-isomers.
Reason : Dipole moments of cis-isomers are higher than trans-isomers.
60. Assertion : The cell potential of mercury cell is 1.35 V, which remains constant.
Reason : In mercury cell, the electrolyte is a paste of KOH and ZnO.
AIIMS MBBS Entrance Exam – 2008
Biology
1. Which one of the following is a matching pair of a certain body feature and its value/count in a normal human adult ?
(a) Urea – 5-10 mg/100 mL of blood
(b) Blood sugar (fasting) – 70-100 mg/100 mL
(c) Total blood volume – 5.6
(d) ESR in Wintrobe method – 9-15 mm in males and 20-34 mm in females
2. Which one of the following is a matching pair ?
(a) Lubb – Sharp closure of AV values at the beginning of ventricular systole
(b) Dup – Sudden opening of semilunar valves at the beginning of ventricular diastole
(c) Pulsation of the radial artery values in the blood vessels
(d) Initiation of the heart beat – Purkinje fibres
3. July 11 is observed as
(a) World population day
(b) No tobacco day
(c) World environment day
(d) World health day
4. Biological Oxygen Demand (BOD) is a measure of
(a) industrial wastes poured into water bodies
(b) extent to which water is polluted with organic compound
(c) amount of carbon mono-oxide inseparably combined with haemoglobin
(d) amount of oxygen needed by green plants during night
5. In almost all Indian metropolitan cities like Delhi, the major atmospheric pollutant (s) is/are
(a) Suspended Particulate Matter (SPM)
(b) oxides of sulphur
(c) carbon dioxide and carbon mono-oxide
(d) oxides of nitrogen
6. People recovering from long illness are often advised to include the alga Sprulina in their diet because it
(a) makes the food easy to digest
(b) is rich in proteins
(c) has antibiotic properties
(d) restores the intestinal microflora
7. Both corpus luteum and macula lutea are
(a) found in human ovaries
(b) a source of hormones
(c) characterized by a yellow colour
(d) contributory in maintaining pregnancy
8. Photochemical smog formed in congested metropolitan cities mainly consists of
(a) ozone, peroxyacetyl nitrate and NOx
(b) smoke, peroxyacetyl nitrate and SO2
(c) hydrocarbons, SO2 and CO2
(d) hydrocarbons, ozone and SOx
9. The plant part which consists of two generations one within the other, is
(a) germinated pollen grain
(b) embryo
(c) unfertilized ovule
(d) seed
10. Cultivation of Bt cotton has been much in the news. The prefix Bt means :
(a) Barium-treated cotton seeds
(b) Bigger thread variety of cotton with better tensile strength
(c) produced by biotechnology using restriction enzymes and ligases
(d) carrying an endotoxin gene from Bacillus thuringiensis
11. Which one feature is common to leech, cockroach and scorpion ?
(a) Nephridia
(b) Ventral nerve cord
(c) Cephalization
(d) Antennae
12. The total number of nitrogenous bases in human genome is estimated to be about
(a) 3.5 million
(b) 35 thousand
(c) 35 million
(d) 3.1 billion
13. The great barrier reef along the east coast of Australia can be categorized as
(a) population
(b) community
(c) ecosystem
(d) biome
14. Which one of the following is a matching pair of a drug and its category?
(a) Amphetamines – stimulant
(b) Lysergic acid dimethyl amide – narcotic
(c) Heroin – psychotropic
(d) Benzodiazepam – pain killer
15. A baby has been born with a small tail. It is the case exhibiting
(a) retrogressive evolution
(b) mutation
(c) atavism
(d) metamorphosis
16. Which one of the following is correctly matched regarding an institute and its location?
(a) National Institute of Virology – Pune
(b) National Institute of Communicable disease – Lucknow
(c) Central Drug Research Institute – Kasauli
(d) National Institute of Nutrition – Mumbai
17. Severe Acute Respiratory Syndrome (SARS)
(a) is caused by a variant of Pneumococcus pneumoniae
(b) is caused by a variant of the common cold virus (corona virus)
(c) is an acute form of asthma
(d) affects non-vegetarians much faster than the vegetarians
18. What is the first step in the Southern blot technique?
(a) Denaturation of DNA on the gel for hybridization with specific probe
(b) Production of a group of genetically identical cells
(c) Digestion of DNA by restriction enzyme
(d) Denaturation of DNA from a nucleated cell such as the one from the scene of crime
19. Which one of the following pairs is correctly matched with regard to the codon and the amino acid coded by it?
(a) UUA-valine
(b) AAA-lysine
(c) AUG-cysteine
(d) CCC-alanine
20. Based on cellular mechanisms there are two major types of regeneration found in the animals. Which one of the following is the correct example of the type mentioned?
(a) Morphollaxis – Regeneration of two transversely cut equal pieces of a Hydra into two small hydras
(b) Epimorphosis – Replacement of old and dead erythrocytes by the new ones
(c) Morphollaxis – Healing up of a wound in the skin
(d) Epimorphosis – Regeneration of crushed and filtered out pieces of a Planaria into as many new Planarians
21. Which one of the following four glands is correctly matched with the accompanying description?
(a) Thyroid – Hyperactivity in young children causes cretinism
(b) Thymus – Starts undergoing atrophy after puberty
(c) Parathyroid – Secretes parathormone, which promotes movement of calcium ions from blood into bones during classification
(d) Pancreas – Delta cells of the islets of Langerhans secrete a hormone, which stimulates glycolysis in liver
22. An insect bite may result in inflammation of that spot. This is triggered by the alarm chemicals such as
(a) histamine and dopamine
(b) histamine and kinins
(c) interferons and opsonin
(d) interferons and histones
23. Which one of the following pairs of geographical areas show maximum biodiversity in our country?
(a) Sunderbans and Rann of Kutch
(b) Eastern Ghats and West Bengal
(c) Eastern Himalaya and Western Ghats
(d) Kerala and Punjab
24. One of the ex situ conservation methods for endangered species is
(a) wild-life sanctuaries
(b) biosphere reserves
(c) cryopreservation
(d) national parks
25. Formation of non-functional methaemoglobin causes blue-baby syndrome. This is due to
(a) excess of arsenic concentration in drinking water
(b) excess of nitrates in drinking water
(c) deficiency of iron in food
(d) increased methane content in the atmosphere
26. Antigen binding site in an antibody is found between
(a) two light chains
(b) two heavy chains
(c) one heavy and one light chain
(d) either between two light chains or between one heavy and one light chain depending upon the nature of antigen
27. Grain colour in wheat is determined by three pairs of polygene. Following the cross AABBCC (dark colour) × aabbcc (light colour), in F2 generation what proportion of the progeny is likely to resemble either parent?
(a) Half
(b) Less than 5 percent
(c) One third
(d) None of these
28. The given graph shows the effect of substrate concentration on the rate of reaction of the enzyme green gram-phosphatase. What does the graph indicate?

(a) The rate of enzyme reaction is directly proportional to the substrate concentration
(b) Presence of an enzyme inhibitor in the reaction mixture
(c) Formation of an enzyme-substrate complex
(d) At higher substrate concentration of pH increases
29. In an experiment, freshly hatched larvae of an insect (khapra beetle) were reared on a basal diet (complete diet without cholesterol) with increasing amounts of cholesterol. Results obtained are shown in the graph given in the table

The graph indicates :
(a) cholesterol is an essential dietary requirement of khapra beetle
(b) growth of khapra beetle is directly proportional to cholesterol concentration
(c) cholesterol concentration of 2 μg/g diet is the optimum level
(d) growth of khapra beetle in inhibited when cholesterol concentration exceeds 2μg/g diet
30. Given below is a trable comparing the effects of sympathetic and parasympathetic nervous system for four features (a-d). Which one feature is correctly described?
(a) Feature – Salivary gland ; Sympathetic Nervous System – Stimulates secretion ; Parasympathetic Nervous System – Inhibits secretion
(b) Feature – Pupil of the eye ; Sympathetic Nervous System – Dilate ; Parasympathetic Nervous System – Constricts
(c) Feature – Heart rate ; Sympathetic Nervous System – Decreases ; Parasympathetic Nervous System – Increases
(d) Feature – Intestinal peristalsis ; Sympathetic Nervous System – Stimulates ; Parasympathetic Nervous System – Inhibits
31. Which one of the following animals is correctly matched with its one characteristic and the taxon?
(a) Animals – Millipede ; Characteristic – Ventral nerve cord ; Taxon – Arachnida
(b) Animals – Duckbilled platypus ; Characteristic – Oviparous ; Taxon – Mammalian
(c) Animals – Silver fish ; Characteristic – Pectoral and pelvic fins ; Taxon – Chordate
(d) Animals – Sea anemone ; Characteristic – Triploblastic ; Taxon – Cnidaria
32. Which one of the following pairs of the kind of cells and their secretion of correctly matched?
(a) Oxyntic cells – A secretion with pH between 2.0 and 3.0
(b) Alpha cells of islets of – Secretion that decreases blood sugar level
(c) Kupffer cells – A digestive enzyme that hydrolyses nucleic acids
(d) Sebaceous glands – A secretion that evaporates for cooling
33. Which of the following pair of feature is a good example of polygenic inheritance?
(a) Human height and skin colour
(b) ABO blood group in humans and flower colour of Mirabilis jalapa
(c) Hair pigment of mouse and tongue rolling in humans
(d) Human eye colour and sickle cell anaemia
34. Mating of an organism to a double recessive in order to determine whether it is homozygous or heterozygous for a character under consideration is called
(a) reciprocal cross
(b) test cross
(c) dihybrid cross
(d) back cross
35. In the following table identify the correct matching of the crop, its disease and the corresponding pathogen
(a) Crop – Citrus ; Disease – Canker ; Pathogen – Pseudomonas rubrilineans
(b) Crop – Potato ; Disease – Late blight ; Pathogen – Fusarium udum
(c) Crop – Brinjal ; Disease – Root-knot ; Pathogen – Meloidogyne incognita
(d) Crop – Pigeon pea ; Disease – Seed gall ; Pathogen – Phytophthora infestans
36. Genes present in the cytoplasm of eukaryotic cells, are found in
(a) mitochondria and inherited via egg cytoplasm
(b) lysosomes and peroxisomes
(c) Golgi bodies and smooth endoplasmic reticulum
(d) plastids and inherited via male gamete
37. A normal woman whose father was colourblind, is married to a normal man. The sons would be
(a) 75% colourblind
(b) 50% colourblind
(c) all normal
(d) all colourblind
38. Which one of the following precedes re-formation of the nuclear envelope during M-phase of the cell cycle?
(a) Decondensation from chromosomes and reassembly of the nuclear lamina
(b) Transcription from chromosomes and reassembly of the nuclear lamina
(c) Formation of the contractile ring and formation of the phragmoplast
(d) Formation of the contractile ring and transcription from chromosomes
39. What is common between chloroplasts, chromoplasts and leucoplasts?
(a) Presence of pigments
(b) Possession of thylakoids and grana
(c) Storage of starch, proteins and lipids
(d) Ability to multiply by a fission-like process
40. In India, we find mangoes with different flavours, colours, fibre content, sugar content and even shelf life. The large variation is ion account of
(a) species diversity
(b) induced mutations
(c) genetic diversity
(d) hybridization
In the following questions (41-60), a statement of Assertion (A) is followed by a statement of Reason (R)
(a) If both Assertion and Reason are true and the Reason is the correct explanation of the Assertion.
(b) If both Assertion and Reason are true but the Reason is not the correct explanation of the Assertion.
(c) If Assertion is true statement but Reason is false.
(d) If both Assertion and Reason are false statements.
41. Assertion : A network of food chains existing together in an ecosystem is known as a food web.
Reason : An animals like kite cannot be a part of a food web.
42. Assertion : In plant tissue culture, somatic embryos can be induced from any plant cell.
Reason : Any animals like kite cannot be a part of a food web.
43. Assertion : The earliest organisms that appeared on the earth were non-green and presumably anaerobes.
Reason : The first autotrophic organisms were the chemo-autotrophs that never released oxygen.
44. Assertion : Escherichia coli, Shigella and Salmonella sp. are all responsible for diarrhoeal diseases.
Reason : Dehydration is common to all types of diarrhoeal diseases and adequate supply of fluids and electrolytes should be ensured.
45. Assertion : In collateral vascular bundles, phloem is situated towards inner side.
Reason : In monocot stem, cambium is present.
46. Assertion : In recombinant DNA technology, human genes are often transferred into bacteria (prokaryotes) or yeast (eukaryote).
Reason : Both bacteria and yeast multiply very fast to form huge population, which express the desired gene.
47. Assertion : Interferons are a type of antibodies produced by body cells infected by bacteria.
Reason : Interferons stimulate inflammation at the site of injury.
48. Assertion : Persons suffering from haemophilia fail to produce blood clotting factor VIII.
Reason : Prothrombin producing platelets in such persons are found in very low concentration.
49. Assertion : Replication and transcription occur in the nucleus but translation occurs in the cytoplasm.
Reason : mRNA is transferred from the nucleus into the cytoplasm where ribosomes and amino acids are available for protein synthesis.
50. Assertion : Human ancestors never used their tails and so the tail expressing gene has disappeared in them.
Reason : Lamarck’s theory of evolution is popularly called theory of continuity of germ plasm.
51. Assertion : Diabetes insipidus is marked by excessive urination and too much thirst of water.
Reason : Anti-diuretic hormone (ADH) is secreted by the posterior lobe of pituitary
52. Assertion : Coavervates are believed to be the precursors of life.
Reason : Coacervates were self-duplicating aggregates of proteins surrounded by lipid molecules.
53. Assertion : Tapeworm roundworm and pinworm and endoparasites of human intestine.
Reason : Improperly cooked food is the source of intestinal infections.
54. Assertion : Fish metal is a rich source of protein for cattle and poultry.
Reason : Fish meal is produced from non-edible parts of fishes like fins, tail.
55. Assertion : Dope test is used to estimate the level of blood alcohol by analyzing the breath of persons drinking alcohol.
Reason : A drunken person usually feels tens and less talkative.
56. Assertion : Mast cells in the human body release excessive amounts of inflammatory chemicals, which cause allergic reactions.
Reason : Allergens in the environment on reaching human body stimulate mast cells in certain individuals.
57. Assertion : Smaller the organism higher is the rate of metabolism per gram weight.
Reason : The heart rate of a six month old baby is much higher than that of an old person.
58. Assertion : Bats and whales are classified as mammals.
Reason : Bats and whales have four-chambered heart.
59. Assertion : Inhabitants close to very busy airports are likely to experience health hazards.
Reason : Sound level of jet aeroplanes usually exceeds 160 dB.
60. Assertion : A cell membrane shows fluid behaviour.
Reason : A membrane is a mosaic or composite of diverse lipids and proteins.
AIIMS MBBS Entrance Exam – 2008
General Knowledge
1. The first of the GAEL (Global Alliance for the elimination of Leprosy) was held in
(a) New Delhi
(b) Bombay
(c) Culcutta
(d) Paris.
2. Megger is an instrument to measure
(a) very low resistance
(b) insulation resistance
(c) inductance of a coil
(d) all of the above
3. Terminator technology promotes sale of which of the following that is/are generated by it?
(a) gransgenic fertile seed.
(b) gene modified plants.
(c) genetically engineered seeds fertilized in next generation.
(d) all of these.
4. Which among the following has become the third tiger reserve of Assam?
(a) Manas Wildlife Sanctury
(b) Kaziranga National Park
(c) Nameri National Park
(d) none of these.
5. To make the acidic soil suitable for agricultural, one of the following material is used.
(a) lime
(b) gypsum
(c) calcium superphosphate
(d) vegetable compost.
6. The landform which is not associated with wind erosion is
(a) sand dune
(b) inselberg
(c) drumlin
(d) mushroom rock.
7. BCG vaccination is to be given to a new born child
(a) immediately after child birth
(b) within 48 hours
(c) within seven days
(d) within six months.
8. For reproducing sound, CD (compact disc) audioplayer uses a
(a) quartz crystal
(b) titanium needle
(c) laser beam
(d) barium titanate ceramic
9. In a three pin electrical plug, longest pin should be connected to
(a) ground terminal.
(b) live terminal.
(c) neutral terminal.
(d) any terminal.
10. The new addition to the commonwealth games 2010 is
(a) shooting
(b) hockey
(c) wrestling
(d) football.
11. The name of the player who established record in World Cup Cricket 2007 of making six sixes is
(a) Sanath Jaisurya
(b) Virendra Sehwag
(c) Adam Gilchrist
(d) Hershelle Gibbs.
12. The lateral meaning of the word Arya is
(a) superior
(b) learned
(c) priest
(d) warrior.
13. When T. V. is switched on
(a) audio and video both start simultaneously
(b) audio is heard immediately but video starts later because video needs some warm up time
(c) video starts immediately but audio is heard later because sound travels at a lesser speed than light
(d) it depends on the T.V. stand.
14. The instrument of music in which Ustad Amjad Ali Khan has distinguished himself is
(a) sarod
(b) violin
(c) sitar
(d) shehnai
15. A deep and narrow river valley with steep bank is called
(a) geyser
(b) bluff
(c) delta
(d) canyon.
16. A ‘breath test’ used by traffic police to check drunken drivers uses –
(a) potassium dichromate-sulphuric acid
(b) potassium permanganate sulphuric acid
(c) turmeric on filter paper
(d) silica gel coated with silver nitrate
17. In which of the following books is ‘Knowledge is Power’ written?
(a) Essay on Man
(b) Paradise Lost
(c) Leviathan
(d) Das Capital.
18. Who said “where weather accumulates, men decay”?
(a) Abraham Lincoln
(b) Mao Tsetung
(c) Karl Marx
(d) Goldsmith
19. Which of the following computer viruses is named after cherry and caffeine soft drink popular with programmers?
(a) sircam
(b) code pink
(c) code red
(d) malisa
20. The fourth Buddist council was held during the reign of
(a) Ashoka
(b) Chandragupta
(c) Kanishka
(d) Chandragupta Vikramaditya