AIIMS 2017 Question Paper
AIIMS 2017 Question Paper got four sections Physics, Chemistry, Biology and General Knowledge. All the questions asked in AIIMS 2017 entrance exam are given here for ready reference of students appearing AIIMS Medical Entrance in India.
AIIMS MBBS Entrance Exam Paper-2017
PHYSICS
1. What will be the a vs x graph for the following graph ?

(a) 
(b) 
(c) 
(d) 
2. The driver of a car travelling with speed 30 ms−1 towards a hill sounds a horn of frequency 600 Hz. If the velocity of sound in air is 330 ms−1, the frequency of reflected sound as heard by driver is
(a) 550 Hz
(b) 555.5 Hz
(c) 720 Hz
(d) 500 Hz
3. An interference pattern is observed by Young’s double slit experiment. If now the separation between coherent source is halved and the distance of screen from coherent source s is doubled, then now fringe width
(a) becomes double
(b) becomes one-fourth
(c) remains same
(d) becomes four times
4. Two capacitor C and C/2n are connected to a battery of V volts, as shown below

The work done in charging both the capacitors fully is
(a) 2CV2
(b) ![]()
(c) ![]()
(d) ![]()
5. A series R-C circuit is connected to AC voltage source. Consider two cases; (A)When C is without a dielectric medium and (B) when C is filled with dielectric of constant 4. The current IR through the resistor and voltage VC across the capacitor are compared in two cases. Which of the following is true ?
(a) ![]()
(b) ![]()
(c) ![]()
(d) None of these
6. A space ship is launched into a circular orbit close to earth’s surface. What additional velocity has now to be imparted to the spaceship in the orbit to overcome the gravitational pull?
(Radius of earth = 6400 km, g = 9.8 m/s2)
(a) 3.28 km/s
(b) 12 km/s
(c) 10 km/s
(d) 40 km/s
7. A force ![]() where k is a positive constant, acts on a particle moving in the x y plane. Starting from the origin, the particle is taken along the positive x-axis to the point (a, 0) and then parallel to the y-axis to the point (a, a). The total work done by the force on the particle is
where k is a positive constant, acts on a particle moving in the x y plane. Starting from the origin, the particle is taken along the positive x-axis to the point (a, 0) and then parallel to the y-axis to the point (a, a). The total work done by the force on the particle is
(a) −2ka2
(b) 2ka2
(c) −ka2
(d) ka2
8. With what minimum acceleration can a fireman slide down a rope while breaking strength of the rope is 2/3 of the weight?
(a) 2/3 g
(b) g
(c) 1/3 g
(d) zero
9. Four blocks of same mass connected by strings are pulled by a force F on a smooth horizontal surface as shown in figure. The tension T1, T2 and T3 will be
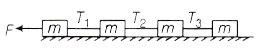
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
10. What is the maximum height attained by a body projected with a velocity equal to one-third of the escape velocity from t he surface of the earth? (Radius of the earth = R)
(a) R/2
(b) R/3
(c) R/5
(d) R/8
11. A block is dragged on a smooth plane with the help of a rope which moves with a velocity v as shown in the figure. The horizontal velocity of the block is
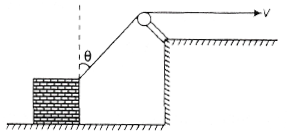
(a) v/sin θ
(b) v sin θ
(c) v/cos θ
(d) v cos θ
12. A tube of sugar solution 20 cm long is placed between crossed nicols and illuminated with light of wavelength 6 × 10−5 If the optical rotation produced is 13° and the specific rotation is 65°, determine the strength of the solution.
(a) 0.1 g/cc
(b) 0.2 g/cc
(c) 0.9 g/cc
(d) 1.0 g/cc
13. A long wire having a semicircular loop of radius r carries a current i as shown in figure. The magnetic induction at the centre O due to entire wire is

(a) ![]()
(b) ![]()
(c) ![]()
(d) None of these
14. Two satellites S1 and S2 are revolving round a planet in coplanar circular orbits of radii r1 and r2 in the same direction, respectively. Their respective periods of revolution are 1h and 8h. The radius of orbit of satellite S1 is equal to 104 What will be their relative speed (in km/h) when they are closest?
(a) π/2 × 104
(b) π × 104
(c) 2π × 104
(d) 4π × 104
15. A body of mass 4 kg moving with velocity 12 m/s collides with another body of mass 6 kg at rest. If two bodies stick together after collision, then the loss of kinetic energy of system is
(a) zero
(b) 288 J
(c) 172.8 J
(d) 144 J
16. The coefficient of cubical expansion of mercury is 0.00018/°C and that of brass 0.00006/° If a barometer having a brass scale were to read 74.5 cm at 30°C, find the true barometric height at 0°C. The scale is supposed to be correct at 15°C.
(a) 74.122 cm
(b) 79.152 cm
(c) 42.161 cm
(d) 142.39 cm
17. The particle of mass m is moving in a circular path of constant radius r such that its centripetal acceleration ac is varying with time t as ac = k2rt2, where k is a constant. The power delivered to particle by the forces acting on it is
(a) 2πmk2r2t
(b) mk2r2t
(c) 1/3mk4r2t5
(d) zero
18. A body of mass 5 × 10−3 kg is launched upon a rough inclined plane making an angle of 30° with the horizontal. Obtain the coefficient of friction between the body and the plane if the time of ascent is half of the time of descent.
(a) 0.346
(b) 0.921
(c) 1.926
(d) 2.912
19. A boy is pushing a ring of mass 3 kg and radius 0.6 m with a stick as shown in figure. The stick applies a force of 3N on the ring and rolls it without slipping with an acceleration of 0.4 m/s2.

The coefficient of friction between the ground and the ring is large enough that rolling always occurs and the coefficient of friction between the stick and ring is F/10. The value of F is
(a) 2 N
(b) 4 N
(c) 6 N
(d) 3 N
20. A load of mass m falls from a height h on the scale pan hung from a spring as shown. If the spring constant is k and mass of the scale pan is zero and the mass m does not bounce relative to the pan, then the amplitude of vibration is

(a) mg
(b) 
(c) 
(d) None of the above
21. In an experiment to measure the height of a bridge by droping stone into water underneath. If the error in measurement of time is 0.2s at the end of 4s, then the error in estimation of height of bridge will be
(neglect the water resistance, i.e. thrust)

(a) ±19.68 m
(b) ±17.22 m
(c) ±7.84 m
(d) ±12.22 m
22. A conductor lies along the z-axis at −5 ≤ Z ≤ 1.5 m and carries a fixed current of 10.0 A in –az direction as shown in figure for a field B = 3 × 10−4 e−0.2x ayT, the total power required to move the conductor at constant speed of x = 2.0 m, y = 0 m in 5 × 10−3 s is
(Assume parallel motion along the x-axis)

(a) 1.57 W
(b) 2.97 W
(c) 4.45 W
(d) 9.87 W
23. A lens of refractive index μʹ. If the focal length of lens in air is f, then its focal length in liquid will be
(a) 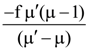
(b) 
(c) 
(d) 
24. A parallel plate capacitor has an electric field of 105 Vm−1 between the plates. If the charge on the capacitor plates is 1 μC, the force on each capacitor plate is
(a) 0.5 N
(b) 0.05 N
(c) 0.005 N
(d) None of these
25. In the given figure, the angle of reflection is

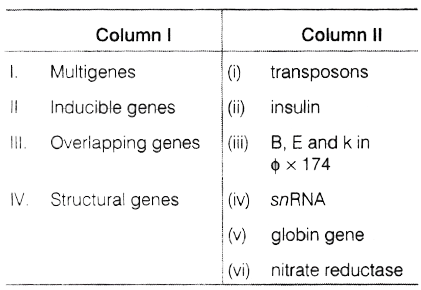
(a) 30°
(b) 60°
(c) 45°
(d) None of these
26. A person of weight 70 kg wants to loose 7 kg by going up and down 12 m high stairs. Assume he burns twice as much fat while going up that going down. If 1 kg of fat is burnt on expending 9000 k-cal. How many times must he go up and down to reduce has 7 kg weight?
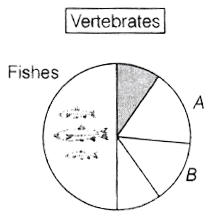
(Take g = 10 ms−2)
(a) 18 × 103 times
(b) 24 × 103 times
(c) 30× 103 times
(d) 21× 103 times
27. The current gain of a transistor in common emitter mode is 49. The change in collector current and emitter current corresponding to change in base curreny by 5.0 μA, will be
(a) 245 μA. 250 μA
(b) 240 μA, 235 μA
(c) 260 μA, 255 μA
(d) None of the above
28. A cylindrical conductor AB of non-uniform area of cross-section carries a current of 5A. The radius of the conductor at one end A is 0.5 cm. The current density at the other end of the conductor is half of the value at A. The radius of the conductor at the end B is nearly
(a) 1.4 cm
(b) 0.7 cm
(c) 0.6 cm
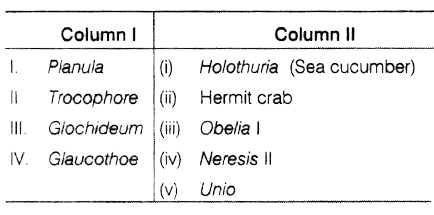
(d) None of these
29. One mole of an ideal diatomic gas undergoes transition from A to B along a path AB as shown below.
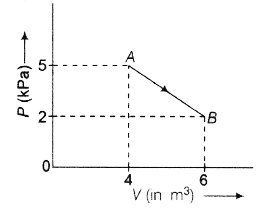
The change in internal energy of the gas during the transition is
(a) 20 kJ
(b) −12 kJ
(c) −20 kJ
(d) 20 J
30. A nuclear explosive is designed to deliver 1 MW power in the form of heat energy. If the explosion is designed with nuclear fuel consisting of U23s to run a reactor at this power level for one year, then the amount of fuel needed is (Given energy per fission is 200 MeV)
(a) 1 kg
(b) 0.01 kg
(c) 3.84 kg
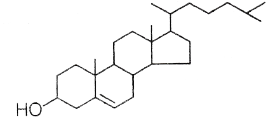
(d) 0.384 kg
31. A thin prism P1 of angle 4° and refractive index 1.54 is combined with another prism P2 of refractive index 1.72 produce dispersion without deviation, the angle of P2 is
(a) 4°
(b) 5.33
(c) 2.6°
(d) 3°
32. The effective resistance between p and q in given figure is

(a) 2Ω
(b) 3Ω
(c) 5Ω
(d) 6Ω
33. Charges +q and –q are placed at points A and B respectively which are a distance 2L apart, C is the mid-point between A and B. The work done in moving a charge +Q along the semicircle CRD is

(a) 
(b) 
(c) 
(d) 
34. In the given figure, C is middle point of line S1S2. A monochromatic light of wavelength λ is incident on slits. The ratio of intensities of S3 and S4 is
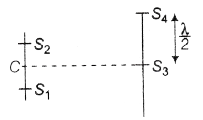
(a) 0
(b) ∞
(c) 4 : 1
(d) 1 : 4
35. A simple telescope, consisting of an objective of focal length 60 cm and a single eye lens of focal length 5 cm is focused on a distant object in such a way that parallel rays emerge from the eye lens. If the object subtends an angle of 2° at the objective, the angular width of the image is
(a) 10°
(b) 24°
(c) 50°
(d) ![]()
36. A specimen of silicon is to be made P-type semiconductor for this one atom of indium, on an average, is doped in 5 × 107 silicon atoms. If the number density of silicon is 5 × 1022 atom/m3 then the number of acceptor atoms per cm3 will be
(a) 2.5 × 1030
(b) 1.0 × 1013
(c) 1.0 × 1015
(d) 2.5 × 1036
37. The angle of dip, if dip needle oscillating in vertical plane makes 40 oscillations per min in a magnetic meridian and 30 oscillations per minute in vertical plane at right angle to the magnetic meridian is
(a) θ = sin−1 (0.5625)
(b) θ = sin−1 (0.325)
(c) θ = sin−1 (0.425)
(d) θ = sin−1 (0.235)
38. Two batteries, one of emf 18V and internal resistance 2Ω and the other of emf 12V and internal resistance 1Ω are connected as shown. The voltmeter V will record a reading of

(a) 14 V
(b) 15 V
(c) 18 V
(d) 30 V
39. The Young’s double slit experiment is performed with blue and green light of wavelengths 4360 Å and 5460 Å If x is the distance of 4th maxima from the central one, then
(a) xblue = xgreen
(b) xblue > xgreen
(c) xblue < xgreen
(d) xblue / xgreen
40. A proper combination of 3 NOT and 1 NAND gates is shown. If A = 0, B = 1, C = 1, then the output of this combination is
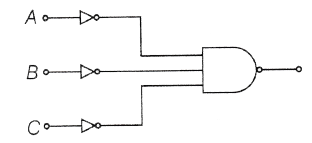
(a) 1
(b) 0
(c) not predictable
(d) None of these
Directions (Q. Nos. 41-60) Read the Assertion and Reason carefully to mark the correct option from those given below.
(a) Both assertion and reason are true and reason is the correct explanation of assertion
(b) Both assertion and reason are true but reason is not the correct explanation of assertion
(c) Assertion is true but reason is false.
(d) Both assertion and reason are false.
41. Assertion For looping a vertical loop of radius, r the minimum velocity at lowest point should be ![]()
Reason In this event the velocity at the highest point will be zero.
42. Assertion A beam of charged particles is employed in the treatment of cancer.
Reason Charged particles on passing through a material medium lose their energy by causing ionization of the atoms along their path.
43. Assertion A spring of force constant k is cut into two pieces having lengths in the ratio 1 : 2. The force constant of series combination of the two parts is 3k/2.
Reason The spring connected in series are represented by k = k1 + k2
44. Assertion The total kinetic energy of a rolling solid sphere is the sum of translational and rotational kinetic energies.
Reason For all solid bodies, total kinetic energy is always twice of translational kinetic energy.
45. Assertion In He-Ne laser, population inversion takes place between energy levels of neon atoms.
Reason Helium atoms have a metastable energy level.
46. Assertion It is hotter over the top of a fire than at the same distance on the sides.
Reason In t he upward direction, the heat propagate through convection.
47. Assertion The average value of alternating emf is 63.39% of the peak value.
Reason The rms value of alternative emf is 70.72% of peak value.
48. Assertion Photoelectric effect can take place only with an electron bound in the atom.
Reason Electron is a fermion whereas proton is a boson.
49. Assertion When θ = 45° or 135°, the value of R remains the same, only the sign changes.
Reason 
50. Assertion In adiabatic expansion the product of p and V always decreases.
Reason In adiabatic expansion process, work is done by the gas at the cost of internal energy of gas.
51. Assertion Cyclotron does not accelerate electron.
Reason Mass of the electron is very small.
52. Assertion The electric field due to a dipole on its axis line at a distance r is E. Then, electric field due to the same dipole on the equatorial line and at the same distance will be E/2.
Reason Electrric field due to dipole varies inversely as the square of the distance.
53. Assertion A potentiometer is preferred over that of a voltmeter for measurement of emf of a cell.
Reason Potentiometer does not draw any current from the cell.
54. Assertion The magnetism of magnet is due to the spin motion of electrons.
Reason Dipole moment of electron is smaller than that due to orbit motion around nucleus.
55. Assertion The mirror used in search lights are parabolic and not concave spherical.
Reason In concave spherical mirror, the image formed is always virtual.
56. Assertion The molecules of a monoatomic gas has three degrees of freedom.
Reason The molecules of diatomic gas has five degrees of freedom.
57. Assertion Corpuscular theory fails in explaining the velocities of light in air and water.
Reason According to corpuscular theory is that light should travel faster in denser media than rarer media.
58. Assertion In α-decay atomic number of daughter nucleus reduces by 2 units from the parent nucleus.
Reason An α-particle carries four units of mass.
59. Assertion Angle of deviation depends on the angle of prism.
Reason For thin prism δ = (μ – 1)A
Where δ = angle of deviation
μ = refractive index, A = angle of prism
60. Assertion Molar heat capacity cannot be defined for isothermal process.
Reason In isothermal process p –V versus T graph is a dot.
AIIMS MBBS Entrance Exam Paper-2017
CHEMISTRY
61. A primary alcohol with a vapour density of 29 contained C = 62.1%, H = 10.3% and reacted with bromine to give a derivative which contains C = 16.5%, H = 2.7% and Br2 = 73.4%. The structural formula of the compound is
(a) CH3CH2CH2OH
(b) CH3CH = CHOH
(c) CH2 = CH – CH2OH
(d) ![]()
62. Total number of optically active forms in molecules with ‘n’ number of asymmetric C-atoms and which are not divisible into two equal halves is
(a) 2n
(b) 2n – 1
(c) ![]()
(d) ![]()
63. The most stable carbocation is
(a) 
(b) 
(c)
(d) 
64.  on ozonolysis gives
on ozonolysis gives
(a) 
(b) 
(c) 
(d) None of these
65. The IUPAC nomenclature of

(a) 2-cyclopentylpropane
(b) 1, 1-dimethyl-1-cyclopentylmethane
(c) 1-(1-methyl) ethylcyclopentane
(d) None of the above
66. For the reaction H2(g) + CO2(g) ⇌ CO (g) + H2O(g), If the initial concentration of [H2] = [CO2] and x mol/L of hydrogen is consumed at equilibrium, the correct expression of kp is
(a) 
(b) 
(c) ![]()
(d) 
67. In the following reaction,

(A) and (B) respectively, are
(a) 
(b) 
(c) 
(d) 
68. In the presence of light, perchlorodiethyl ether is obtained from diethyl ether.
![]()
The mechanism through which this reaction proceed.
(a) Addition mechanism
(b) Substitution mechanism
(c) Free radical mechanism
(d) Elimination mechanism
69. For the reaction,
![]()
pH values ‘x’ and ‘y’ can be
(a) 4 and 5
(b) 4 and 8
(c) 8 and 4
(d) 8 and 9
70. H+ ion always get associated with other atoms or molecules. This is because
(a) ionization enthalpy of hydrogen resembles that of alkali metals.
(b) its reactivity is similar to halogens.
(c) it resembles both alkali metals and halogens.
(d) loss of an electron from hydrogen atom results in a nucleus of very small size as compared to other atoms or ions.
71. Which of the following curve best explains the Freundlich adsorption isotherm?
(a) 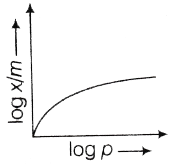
(b) 
(c) 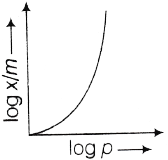
(d) 
72. ![]() (b) hydroboration oxidation (c)
(b) hydroboration oxidation (c)
In the above reaction (a) and (C) are
(a) identical
(b) positional isomers
(c) functional isomers
(d) optical isomers
73. Consider the following reactions

Here, X, Y and Z respectively, are
(a) XeF2, XeF6, XeF4
(b) XeF2, XeF4, XeF6
(c) XeF4, XeF2, XeF6
(d) XeF6, XeF4, XeF2
74. In the equation, 4M + 8CN− + 2H2O + O2 → 4[M(CN)2]− + 4OH−
Identify the metal (M).
(a) Au
(b) Fe
(c) Zn
(d) Cu
75. CuSO4 ∙ 5H2O is blue in colour while CuSO4 is colourless, because
(a) H2O is strong field ligand than SO42−
(b) SO42 is a strong field ligand
(c) CuSO4 cannot form the complex
(d) NO d-d transition is possible in CuSO4
76. Consider the following equation

The end product for the given sequence of reaction is
(a) 
(b) 
(c) 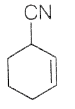
(d) 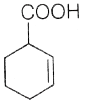
77. Which of the following arrangement correctly shows the magnetic moment of anti-ferromagnetic substance?
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
78. Consider the below given figure.

The correct option for the above presentation is
(a) activation energy of forward reaction is E1 + E2 and product is less stable than reactant
(b) activation energy of forward reaction is E1 + E2 and product is more stable than reactant
(c) activation energy for both forward and backward reaction is E1 + E2 and reactant is more stable than product
(d) activation energy for the backward direction is E1 and product is more stable than reactant
79. In formation of NO+ from NO, the electron is removed from
(a) a σ-orbital
(b) a π-orbital
(c) a σ* -orbital
(d) a π* -orbital
80. The nitrogen base which is present in RNA and absent in DNA is
(a) 
(b) 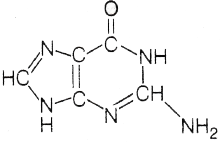
(c) 
(d) 
81. Which of the following reagent is used to distinguish phenol and benzoic acid?
(a) Aqueous NaOH
(b) Tollen’s reagent
(c) Molisch reagent
(d) Neutral FeCl3
82. 5g of a mixture of NaOH and KOH were dissolved and made up to 250 mL. 25 mL of this solution were completely neutralized by 17 mL of (N/2) HCl solution. Then, the percentage of KOH in mixture is
(a) 80
(b) 10
(c) 34
(d) 56
83. CO2 gas along with solid (Y) is obtained when sodium salt (X) is heated. (X) is again obtained when CO2 gas is passed into aqueous solution (Y). (X) and (Y) respectively, are
(a) Na2CO3, Na2O
(b) Na2CO3, NaOH
(c) NaHCO3, Na2CO3
(d) Na2CO3, NaHCO3
84. In van der Waal’s equation of state for non-ideal gas, the term that accounts for intermolecular force is
(a) (V = b)
(b) (RT)−1
(c) 
(d) RT
85. Rat constant (K) varies with temperature as given by equation
![]()
Consider the following about this equation
(I) Pre exponential factor is 105.
(II) Ea is 9.212 kcal.
(III) Variation of log K with 1/T is linear.
Select the correct statement.
(a) I, II and III
(b) Both I and II
(c) Both II and III
(d) Both I and III
86. In the following reaction,

Compounds (A) and (B) respectively are
(a) Nitrobenzene and fluorobenzene
(b) Phenol and benzene
(c) Benzene diazonium chloride and fluorobenzene
(d) Nitrobenzene and chlorobenzene
87. The basic strength of

will be in order
(a) I < II < III
(b) II < III < I
(c) III < II < I
(d) III < I < II
88. Which of the following sets contain only addition polymers?
(a) Polyethylene, polypropylene, terylene
(b) Polyethylene, PVC, teflon
(c) Buna-S, nylon, polybutadiene
(d) Bakelite, PVC, polyethylene
89. Which of the following has maximum number of unpaired d-electrons?
(a) Fe2+
(b) Cu+
(c) Zn
(d) Ni3+
90. Which of the following conversions involve change in both hybridization and shape?
(a) CH4 → C2H6
(b) NH3 → NH4+
(c) BF3 → BF4−
(d) H2O → H3O+
91. We have three aqueous solutions of NaCl labelled as (A), (B) and (C) with concentration of 0.1 M, 0.01 M and 0.001 M, respectively. The value of van’t Hoff factor of these solutions will be in order
(a) iA < iB < iC
(b) iA < iB < iC
(c) iA = iB = iC
(d) iA < iB > iC
92. Which transtition in the hydrogen atomic spectrum will have the same wavelength as the Balmer transition (i.e. n = 4 to n = 2) of He+ spectrum?
(a) n = 4 to n = 3
(b) n = 3 to n = 2
(c) n = 4 to n = 2
(d) n = 2 to n = 1
93. Consider the following equation,

Here, product ‘A’ is
(a) 
(b) 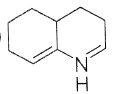
(c) 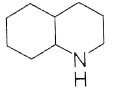
(d) 
94. Among the following, the true statements are
(I) PH5 and BiCl5 do not exist.
(II) pπ-dπ is present in SO2.
(III) electrons travel with speed of light.
(IV) SF4 and CH4 has same shape.
(V) I3+ has bent shape.
(a) I, III
(b) I, II, V
(c) I, III, V
(d) I, II, IV
95. What is freezing point of solution containing 8.1 g of HBr in 100 g of water, assuming the acid to be 90% ionized.
(Kf for water = 1.86 kg mol−1 and molar mass of HBr = 81)
(a) −0.35°C
(b) −1.35°C
(c) −2.35°C
(d) −3.35°C
96. pH of solution of a strong acid is 5.0. What will be the pH of solution obtained after diluting the given solution to 100 times?
(a) 5.8
(b) 6.7
(c) 9.3
(d) 13
97. If at 298 K, the bond energies of C – H, C – C, C = C and H – H bonds are respectively 414, 347, 615 and 435 kJ mol−1, the value of enthalpy change for the reaction ; H2C = CH2 + H2(g) → H3C – CH3 (g) at 298 K, will be
(a) +250 kJ
(b) −250 kJ
(c) + 125 kJ
(d) −125 kJ
98. The number of coulombs required to reduce 12.3 g of nitrobenzene to aniline is
(a) 96500 C
(b) 5790 C
(c) 95700 C
(d) 57900 C
99. If 0.5 moles of BaCl2 is mixed with 0.2 moles of Na3PO4, the maximum number of moles of Ba3(PO4)2 that can be formed is
(a) 0.7
(b) 0.5
(c) 0.03
(d) 0.10
100. For the reaction, A2(g) + 4B2(g) ⇌ 2AB4(g), ∆H < 0, the formation of AB4 will be favoured at
(a) low temperature, high pressure
(b) high temperature, low pressure
(c) low temperature, low pressure
(d) high temperature, high pressure
Directions (Q. Nos. 101-120) In the following questions a statements of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choice.
(a) Both Assertion and Reason are true and Reason is the correct explanation of Assertion
(b) Both Assertion and Reason are true but Reason is not the correct explanation of Assertion
(c) Assertion is true but Reason is false
(d) Both Assertion and Reason are false
101. Assertion (A) Ecell increases with increase in concentration of Ag+
Reason (R) Ecell has positive value.
102. Assertion (A) London smong is produced when carbon soot particles combine with gaseous oxides of sulphur.
Reason (R) Presence of carbon particles and SO2 makes it reducing in nature.
103. Assertion (A) Buffer solution are composed of strong acids and strong bases.
Reason (R) It maintain the pH to a constant value of 7.4.
104. Assertion (A) Mg continues to burn in nitric oxide.
Reason (R) During the burning, the heat evolved does not decompose NO.
105. Assertion (A) Superoxides of alkali metals are paramagnetic in nature.
Reason (R) Superoxide contain the ion which has one unpaired electron.
106. Assertion (A) The ionization of hydrogen sulphide in water is low in t he presence of HCl.
Reason (R) H2S is a weak acid.
107. Assertion (A) Aniline on reaction with NaNO2/HCl at 0°C followed by coupling with β-naphthol gives a dark blue coloured precipitate.
Reason (R) The colour of the compound formed in the reaction of aniline with NaNO2/HCl at 0°C followed by coupling with β-naphthol is due to the extended conjugation.
108. Assertion (A) Presence of nitro group facilitates nucleophilic substitution reactions in aryl halides.
Reason (R) The intermediate carbocation is stable due to presence of nitro group.
109. Assertion (A) Glucose gives reddish brown precipitate with Fehling solution.
Reason (R) Reaction of glucose with Fehling solution gives CuO and gluconic acid.
110. Assertion (A) The following molecule is non-superimposable on its mirror image, hence it is chiral.

Reason (R) All chiral molecules have chiral centers.
111. Assertion (A) Pb4+ compounds are stronger oxidizing agents than Sn4+
Reason (R) The higher oxidation state for group-14 elements are more stable for the heavier members of the group due to inert pair effect.
112. Assertion (A) Aryl halides undergo nucleophilic substitution with ease.
Reason (R) Hybridization of C-atom attached to halide is sp3-hybrid.
113. Assertion (A) Both vapour pressure and boiling point depends on surface area of the liquid.
Reason (R) Higher the surface area, lower be the boiling point whereas higher will be the vapour pressure.
114. Assertion (A) Black body is an ideal body that emits and absorb radiations of all frequencies.
Reason (R) The frequency of radiations emitted by a body goes from lower frequency to higher frequency with an increase in temperature.
115. Assertion (A) Separation of Zr and Hf is difficult.
Reason (R) Zr and Hf lie in the same group of the periodic table.
116. Assertion (A) ∆Hmixing and ∆Vmixing for non-ideal solution with +ve deviation is zero.
Reason (R) A – B interaction is more than that between A – A and B – B.
117. Assertion (A) It is impossible to determine the exact position and exact momentum of an electron, simultaneously.
Reason (R) The path of an electron is clearly defined.
118. Assertion (A) The central atom of NH3 and H2O, are both sp3-hybridized yet H – N – N bond angle is greater than that of H – O – H.
Reason (R) In NH3, N-atom has one lone pair of electron whereas in H2O, oxygen atom has two low pairs of electrons.
119. Assertion (A) Complexes of MX6 and MX5L type (X and L are unidentate) do not show geometrical isomerism.
Reason (R) Geometrical isomerism is not shown by complexes of coordinate number –6.
120. Assertion (A) The configuration of is z.
Reason (R) z-configuration shows the presence of bulkier groups at the opposite side of double bond.
AIIMS MBBS Entrance Exam Paper-2017
BIOLOGY
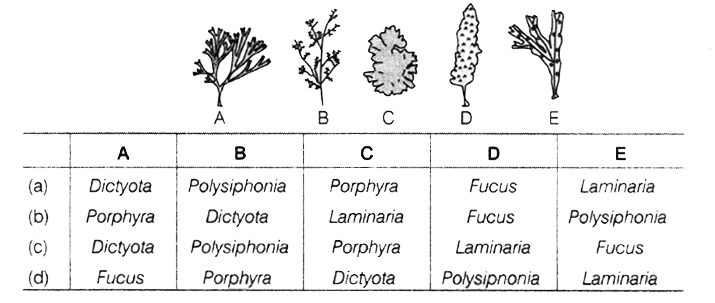
121. In the given diagrams, some of the algae have been labelled as A, B, C and E. Choose the correct option to identify these algae.
122. Read the following statement regarding bacteria.
(I) Bacteria exchange their genetic matter through conjugation which involve cell to cell contact.
(II) Transduction in ‘Salmonella is reported by Tatum and Lederberg in 1952.
(III) Citrus canker disease is caused by bacteria Xanthomonas citri.
(IV) Hans Christian gram’s staining method is based on cell wall composition of bacteria.
Choose the correct option with true statements
(a) I and III
(b) I, III and IV
(c) I, II and III
(d) II and IV
123. Among which of the animals urinary bladder is absent?
(a) Frog
(b) Crow
(c) Snake
(d) Camel
124. An example of gene therapy is
(a) production of injectable hepatitis-B vaccine
(b) production of vaccines in food crops like potatoes which can be eaten
(c) introduction of gene for adenosine deaminase in persons suffering from Severe Combined Immuno Deficiency (SCID)
(d) production of test tube babies by artificial insemination and implantation of fertilized eggs
125. In pea plants, green pod colour is dominant over yellow pods. 1000 seeds taken from a pea plant on germination produces 760 green pod and 240 yellow pod plants. The parental genotype and phenotype of the seed plant are
(a) heterozygous and yellow
(b) heterozygous and green
(c) homozygous and yellow
(d) homozygous and green
126. Identify the incorrect option for effects of the red and far red light.

127. Classical Taxonomy is based on
(a) morphological traits
(b) habitat of organisms
(c) similarities and dissimilarities of behaviour
(d) phylogeny
128. Which of the following factor is incorrect for the low levels of immune response during Plasmodium infection?
(a) Different types of antigens are expressed at varying stages of Plasmodium life cycle
(b) The stages during its life cycle are mostly intracellular
(c) The sporozoites of plasmodium are rapidly cleared from blood circulation
(d) Plasmodium infection primarily destroys the macrophages and dendritic cells present in blood
129. Heterocyst present in Nostoc is specialized for
(a) fragmentation
(b) nitrogen-fixation
(c) symbiotic relation
(d) food storage
130. Which of the following plant growth hormone increases the yield of sugar by increasing the length of stem in sugarcane?
(a) Cytokinin
(b) Ethylene
(c) Gibberellic acid
(d) Auxin
131. Match the following columns
(a) I – (i); II – (iii); III – (v); IV – (ii)
(b) I – (v); II – (vi); III – (iii); IV – (ii)
(c) I – (iv); II – (v); III – (vi); IV – (i)
(d) I – (i); II – (ii); III – (v); IV – (vi)
132. Identify the correct matched pair
(a) Exchange of segments of chromatids-Zygotene
(b) Terminalization of chiasmata-Diakinesis
(c) Appearance of chiasmata-Leptotene
(d) Synapsis of homologous chromosomes-Diplotene
133. The cavity of diencephalon is known as
(a) first ventricle
(b) second ventricle
(c) third ventricle
(d) fourth ventricle
134. The modified equation for water potential is
(a) Ψw = Ψs + Ψp
(b) Ψw = Ψs – Ψp
(c) Ψw = Ψs
(d) Ψw = Ψp – Ψs
135. Which one of the following option is correct regarding digestion of food substrates?

136. Refer to the following figure represent global biodiversity. Identify A-E and choose the correct option.


(a) A – Birds ; B – Reptiles ; C – Algae ; D – Molluscs ; E – Mosses
(b) A – Mammals ; B – Birds ; C – Lichens ; D – Molluscs ; E – Mosses
(c) A – Birds ; B – Amphibians ; C – Mosses ; D – Insects ; E – Algae
(d) A – Birds ; B – Reptiles ; C – Algae ; D – Insects ; E – Mosses
137. Gemmule formation is a common mode of asexual reproduction in
(a) Paramecium
(b) Hydra
(c) sponges
(d) yeast
138. Which one is an incorrect match?
(a) Glucoma-Abnormal high pressure on liquid of eye
(b) Eustachian tube-Connects middle ear cavity with pharynx
(c) Caloreceptor-Heat
(d) Interoreceptor-Touch
139. Which of the following is not used as a biopesiticide?
(a) Bacillus thuringiensis
(b) Xanthomonas campestris
(c) Nuclear Polyhedrosis Virus (NPV)
(d) Trichoderma horzianum
140. Which one of the following option is not correctly matched?

141. Which one option is incorrectly matched regarding biological magnification of DDT in aquatic ecosystem.
(a) Small fish – 0.5 ppm
(b) Large fish – 2 ppm
(c) Fish-eating birds – 25 ppm
(d) Zooplankton – 0.003 ppm
142. The desert grasses, often curls their leaf to minimize water loss due to presence of
(a) spines
(b) palisade parenchyma
(c) bundle sheath cells
(d) bulliform cells
143. Identify the correct matches for crops and their improved varities

144. Refer to the following figures.

Match the following columns and choose the correct option from the codes given below.

(a) A -3-II; B-1-III; C-2-I
(b) A -1-II; B-3-I; C-2-I
(c) A -2-III; B-1-II; C-3-I
(d) A -3-I; B-2-II; C-1-III
145. Which part of ovary in mammals acts as an endocrine gland after ovulation?
(a) Graafian follicle
(b) Vitelline membrane
(c) Germinal epithelium
(d) Chorion
146. From the statements given below, which one most likely represents and example of disruptive selection.
(I) Industrial melanism in peppered moth.
(II) Population of butterflies that are either all yellow or all blue.
(III) Population of rabbits that evolves more body fat in response to a cold climate.
(IV) Population of wrens that evolves to be smaller at sexual maturity in response to predation pressure.
(V) Very tall and very short pine trees being removed from a population by herbivorey.
(a) Only II
(b) II and III
(c) Only IV
(d) III and IV
147. The ‘amino acid derivative’ among the following hormone is
(a) insulin
(b) testosterone
(c) oestradiol
(d) epinephrine
148. Identify the incorrect match from those given below

149. Which one is correct sequence occurring in glycolysis?
(a) G-6-P→ PEP → 3-PGAL → 3-PGA
(b) G-6-P → 3-PGAL → 3-PGA → PEP
(c) G-6-P → PEP → 3-PGA → 3-PGAL
(d) G-6-P → 3-PGA → 3-PGAL → PEP
150. Match the larval stages (in column I) with there corresponding animals (in column II) and select the correct option.
(a) I – (i); II – (iii); III – (v); IV – (iv)
(b) I – (iii); II – (iv); III – (v); IV – (ii)
(c) I – (i); II – (ii); III – (iii); IV – (iv)
(d) I – (iii); II – (i); III – (ii); IV – (v)
151. Adenosine deaminase (ADA deficiency) could be permanently cure, if the gene isolated from marrow cells producing ADA is introduced into cell at
(a) early embryonic stages
(b) Late embryonic stages
(c) early childhood
(d) None of the above
152. A man whose father was colourblind marries a woman, who had a colourblind mother and normal father. What percentage of male children of this couple will be colourblind?
(a) 25%
(b) 0%
(c) 50%
(d) 75%
153. The contraction of the muscle continues in sliding filament theory
(a) till ATP binds to myosin head
(b) till ADP binds to myosin head
(c) till Ca2+ is present in sarcoplasm
(d) till polymerization of myosin head is going on
154. Mitochondria and chloroplast are believed to be bacterial endoymbiont because
(I) they have self nucleic acid i.e., circular ds, DNA and RNAs
(II) 70s ribosomes
(III) their membrane resembles that of bacteria, having pour proteins.
(IV). ETS and ATP forming machienary is present.
(a) I and II
(b) I, II and III
(c) All of these
(d) I and IV
155. Which of the following process of urine formation takes place all along the renal tubule and collecting duct?
(a) Ultrafiltration and tubular reabsorption
(b) Ultrafiltration and tubular secretion
(c) Tubular reabsorption and secretion
(d) Anti-current mechanism and reabsorption
156. Oxyhaemogloblin dissociates into oxygen and deoxyhaemoglobin at
(a) low O2 pressure in tissue
(b) high O2 pressure in tissue
(c) equal O2 pressure inside and outside tissue
(d) all times irrespective of O2 pressure
157. The premature termination of polypeptide synthesis due to stop codon can be overcome via compensatory mutation in tRNA. This genetic phenomenon is called
(a) extragenic suppression
(b) intragenic suppression
(c) codon bias
(d) true reversion
158. The best description of natural selection is
(a) the survival of the fittest
(b) the struggle for existence
(c) the reproductive of t he members of a population best adapted to the environment
(d) a change in the proportion of variations within a population
159. The given below figure shows a generalized life cycle of a fungus. Identify A, B and C from the given option.

(a) A – Meiosis ; B – Fertilization ; C – Meiosis
(b) A – Meiosis ; B – Mitosis ; C – Fertilization
(c) A – Mitosis ; B – Meiosis ; C – Fertilization
(d) A – Mitosis ; B – Fertilization ; C – Meiosis
160. The first heart sound occurs due to
(a) opening of semilunar valve
(b) closing of semilunar valve
(c) onset of auricular systole
(d) sudden closure of AV valves
Directions (Q. Nos. 161-180) Read the Assertion and Reason carefully to mark the correct option from those given below
(a) Both Assertion and Reason are true and Reason is the correct explanation of Assertion
(b) Both Assertion and Reason are true, but Reason is not the correct explanation of the Assertion
(c) Assertion is true, but Reason is false.
(d) Both Assertion and Reason are false.
161. Assertion The structure given is the most important animal sterois which is insoluble in water and chemically unreactive
Reason It is important because it is a structural component of cells.
162. Assertion Allelopathy is a form of ammensalism that occurs in plants.
Reason Allelopathy is the symbiotic association of the roots of higher plant and fungi.
163. Assertion Endosperm is formed by the fusin of one male gamete and two polar nuclei.
Reason In pea, the endosperm remains the part of seed as it is not completely used up during embryo development.
164. Assertion In four ‘O’ clock plant, a cross between homozygous white flower and homozygous red flower produce pink flower.
Reason In these plants, the colour of flower is determine by three alleles.
165. Assertion Gross primary productivity is always more than net primary productivity.
Reason Consumers exhibit secondary productivity in a ecosystem.
166. Assertion Gene bank is a type of ex-situ conservation of biodiversity.
Reason It involves maintaining stocks of viable seeds, living growing plants, tissue culture, etc.
167. Assertion Darwin held that small favourable variations formed raw material for evolutions.
Reason Darwin did not study the factors which produce variations.
168. Assertion Claspers of cartilagous fishes are analogous to human penis.
Reason Both acts as a copulatory organs and transfer the sperms into female.
169. Assertion The technique shown in the given figure is frequently used in prenatal disease treatment.

Reason This technique cannot be used for sex-determination of child.
170. Assertion In syconous fruit, the achenes formed are fewer than the total number of flower in the inflorescence.
Reason Upper and middle flower cannot develop into fruits.
171. Assertion The structure given below contains 1-4α-glycosidic bonds.
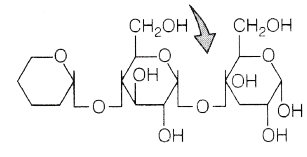
Reason This is a polysaccharide and have right end as reducing end and its left end is called the non-reducing end.
172. Assertion Techniques called superovulation and embryo transplantation are used for cattle improvement.
Reason Gonadotropin injection release more than one ova (superovulation) in high yielding cows. These are fertilized by artificial insemination with sperms from a pedigree bull. Early embryos are transplanted into surrogate mothers for development.
173. Assertion Besides curdling of milk, LAB also improve its nutritional quality by increasing vitamin-B12
Reason LAB check disease causing microbes when it is present in human stomach.
174. Assertion Urinary bladder is lined by transitional epithelium.
Reason Transitional epithelium keeps the size of the urinary bladder constant at all time.
175. Assertion Gall stone are caused by disturbances in the composition of the bile.
Reason A change in the ratio of liver glycogen and bile salts may result in formation of deposits.
176. Assertion Arteries pump blood away from the heart at very low pressure.
Reason Arteries have thin wall with large lumen.
177. Assertion Arival of an impulse at the axon terminal stimulates the release of neurotransmitters in synaptic cleft.
Reason These neurotransmitters are responsible for the opening of ion channels.
178. Assertion Capillary water is the readily available soil water to plants.
Reason Capillary water is the thin film of water which is retained around soil particles.
179. Assertion Hexokinase require divalent cation Mg2+.
Reason Mg2+ or Mn2+ combines with ATP to form MgATP2+.
180. Assertion Agrobacterium tumefaciens is a popular genetic engineer because this bacteria is associated with the roots of all cereal and pulse crops.
Reason A gene incorporated in the bacterial chromosomal genome gets automatically suppressed into the crop with which the bacteria is associated.
AIIMS MBBS Entrance Exam Paper-2017
GENERAL KNOWLEDGE
181. Which of the following is a river flowing from Central India and joining Yamuna/Ganga?
(a) Ganga
(b) Gomti
(c) Kosi
(d) Betwa
182. Which of the following is essential for blood clotting?
(a) RBC
(b) WBC
(c) Blood platelets
(d) Lymphs
183. Who was the man of the match of the 1983 World Cup Final?
(a) Kapil Dev
(b) Roger Binny
(c) Sunil Gavaskar
(d) M Amarnath
184. What is the middle name of the world class batsman Sachin Tendulkar?
(a) Rohan
(b) Ramesh
(c) Rahul
(d) Ravi
185. With which game is the Agha Cup associated?
(a) Football
(b) Cricket
(c) Basketball
(d) Hockey
186. Miss Universe 1995, Ms Sushmita Sen hails from which of the following cities?
(a) Mumbai
(b) Chandigarh
(c) Bengaluru
(d) New Delhi
187. Of the following, which one is the liest insulator?
(a) Wood
(b) Cloth
(c) Glass
(d) Paper
188. The constitution names our country as
(a) Bharat
(b) India, that is Bharat
(c) Hindustan
(d) Aryavarta
189. The first state to become bifurcated after independence was
(a) Bengal
(b) Mumbai
(c) Punjab
(d) Assam
190. Who presides over the meetings of the Rajya Sabha?
(a) President
(b) Vice President
(c) Prime Minister
(d) Speaker
191. Shiv Kumar Sharma is a famous player of
(a) sitar
(b) flute
(c) tabla
(d) santoor
192. Who is Taniya Sachdev?
(a) Dancer
(b) Chess player
(c) Cricketer
(d) Actress
193. Sanjay Dutt, a noted film actor was held under
(a) Act 302
(b) Anti Defection Act
(c) TADA
(d) None of these
194. The Chameli Devi Award is given to an outstanding woman who is
(a) vocalist
(b) lawyer
(c) journalist
(d) scientist
195. Who has won the greatest number of Oscars in his life time?
(a) Ingrid Bergman
(b) David Leon
(c) Charlie Chaplin
(d) Wall Disney
196. Dronacharya Award is given to
(a) fire fighting operation
(b) archery
(c) significant contribution in the spread of literacy
(d) outstanding coaching of athletics
197. Leander Paes is associated with
(a) football
(b) cricket
(c) badminton
(d) tennis
198. How many players are there in a kabaddi team?
(a) 7
(b) 11
(c) 9
(d) 5
199. The famous novel ‘Pride and Prejudice’ is written by
(a) RL Stevenson
(b) George Eliot
(c) Charles Dickens
(d) Jane Austen
200. Who wrote the book ‘Gandhi and Stalin’?
(a) Rajmohan Gandhi
(b) Nelson Mandela
(c) Louis Fisher
(d) Martin Luther