Pharmaceutical Science (PY) Competitive Examination-2012
(GPAT, CET, NIPER etc.)
1. Which of the following respective Phase-I and Phase-II reactions are the most common drug biotransformation reactions?
(A) Oxidation and Glucuronidation
(B) Reduction and Acetylation
(C) Hydrolysis and Glucuronidation
(D) Oxidation and Glutathion conjugation
2. Which one of the following drugs has positive inotropic and negative chronotropic action?
(A) Dopamine
(B) Epinephrine
(C) Digoxin
(D) Isoprenaline
3. Which one of the following therapeutic classes has been proved clinically as a first line therapy for heart failure and has shown decreased hospitalization, improved symptoms and delayed disease progression?
(A) Cardiac glycosides
(B) ACE Inhibitors (ACEIs)
(C) Renin Antagonists
(D) Nitrites
4. Which one of the following glucose transporters is the new drug target for the management of Type-2 diabetes mellitus?
(A) Sodium glucose linked transporter-2 (SGLT2)
(B) Glucose transporter-1 (GLUTI)
(C) Sodium glucose linked transporter-1 (SGLT1)
(D) Glucose transprter-2 (GLUT2)
5. Which one of the following modes of HIV transmission carries highest relative risk of infection with single exposure?
(A) Transfusion of blood and blood products
(B) Perinatal-from mother to child
(C) Sexual contacts with infected partners
(D) Syringe sharing with drug addicts
6. Which of the following are the critical neuro-transmitters playing major role in depression?
(A) Acetylcholine, Norepinephrine and Dopamine
(B) Dopamine, Norepinephrine and Serotonin
(C) Serotonin, Dopamine and y-Amino butyric acid
(D) Acetylcholine, Serotonin and y-Amino butyric acid
7. A 55 years old man is under DOTS treatment for pulmonary tuberculosis for the last four months. Now, he has developed symptoms of peripheral neuritis. Which one of the following is the right addition to his therapy to manager peripheral neuritis α ?
(A) Cyanocobalamin
(B) α-Lipoic acid
(C) Pyridoxine
(D) Prednisolone
8. What is the primary mechanism of action of local anesthetics?
(A) Activation of ligand-gated potassium channels
(B) Blockade of voltage-gated sodium channels
(C) Stimulation of voltage-gated N-type calcium channels
(D) Blockade of GABA-gated chloride channels
9. Which one of the following anti-asthmatic drugs can cause convulsions and arrhythmia?
(A) Prednisolone
(B) Salmeterol
(C) Zafirlukast
(D) Theophylline
10. Which one of the following anti-arrhythmic drugs acts by inhibiting potassium, sodium and calcium channels?
(A) Quinidine
(B) Lignocaine
(C) Amiodarone
(D) Flecainide
11. A 48 years old woman is having the symptoms of weight gain, cold intolerance, constipation, brady-cardia, puffy face, lethargy and dry skin. These symptoms are suggestive of which of the followings?
(A) Over use of corticosteroid
(B) Hypothyroidism
(C) Estrogen deficiency
(D) Over use of thyroxin sodium
12. Increased risk of hypoglycemia and weight gain is the common side effect of drugs used in the management of Type-2 diabetes mellitus. Followings are some commonly used drugs, alone or in combination, for the management of Type-2 diabetes mellitus:
(P) Metformin (Q) Pioglitazone
(R) Lipizide (S) Sitagliptin
Choose the correct combination which is weight neutral and without risk of hypoglycemia:
(A) P and Q
(B) Q and R
(C) R and S
(D) P and S
13. Which one of the following receptors is NOT a ligandgated ion channel receptor?
(A) Nicotinic Receptor
(B) 5HT3-Receptor
(C) GABAA-Receptor
(D) H2-Receptor
14. Which one of the following classes of drugs causes side effects like dryness of mouth, tachycardia, urinary retention, constipation, blurring of vision, precipitation of glaucoma, drowsiness and impairment of cognition?
(A) Anti-adrenergic
(B) Anti-cholinergic
(C) Anti-serotonergic
(D) Anti-dopaminergic
15. Which of the following cytokines are the most important regulators in inflammation and are the targets for anti-inflammatory agents used in rheumatoid arthritis?
(A) Tumor necrosis factor-a and Interleukin-1
(B) Acetylcholine esterase and Eicosanoids
(C) Leukotrienes and Isoprostanes
(D) Adhesion factor and Monoamine oxidase A
16. Which one of the following is a FALSE statement for competitive antagonists?
(A) They have an affinity for the agonist binding site on receptor
(B) They have no intrinsic activity
(C) They cause parallel rightward shift of the control dose response curve
(D) Maximum response of the agonist cannot be achieved in their presence by increasing the concentration of the agonist.
17. Atypical antipsychotics differ from the typical anti-psychotics in various ways that define them as Atypical. Which one of the following is NOT a defining property of the Atypical antipsychotics?
(A) Sustained hyperprolactinemia
(B) Improved efficacy in treating the negative symptoms
(C) Lower risk for extrapyramidal side effects (EPSs)
(D) Greater serotonin receptor blockade than dopamine blockade
18. Which one of the following drugs produces significant relaxation of both venules and arterioles?
(A) Hydralazine
(B) Minoxidil
(C) Diazoxide
(D) Nitroprusside
19. Antiviral action of purine analogues is primarily related to the followings:
(P) Inhibition of RNA synthesis
(Q) Inhibition of DNA polymerase
(R) Immunomodulation
(S) Inhibition of viral penetration
Choose the correct option:
(A) R is correct and Q is incorrect
(B) Q is correct and S is incorrect
(C) P is correct and R is incorrect
(D) S is correct and P is incorrect
20. All of the given four drugs are sympathomimetics:
(P) Adrenaline (Q) Isoprenaline
(R) Pehnylephrine (S) Noradrenaline
Choose the correct statement related to their effects on blood pressure:
(A) P and Q increase systolic and diastolic blood pressure
(B) Q and R increase systolic and diastolic blood pressure
(C) R and S increase systolic blood pressure
(D) P and S increase systolic and diastolic blood pressure
21. All of the given four drugs are neuromuscular blocking agents:
(P) Gallamine (Q) Succinylcholine
(R) Vecuronium (S) d-Tubocurarine
Choose the correct statement about them.
(A) P and Q are competitive neuromuscular blocking agents
(B) Q and R are competitive neuromuscular blocking agents
(C) R and S are non-competitive neuromuscular blocking agents
(D) P and S are competitive neuromuscular blocking agents
22. Which one of the following is a tyrosine kinase inhibitor indicated for a variety of malignancies?
(A) Imatinib
(B) Paclitaxel
(C) Ezetimibe
(D) Mitomycin
23. Which one of the following is the most likely positive sign of pregnancy when detected in urine?
(A) Estrogens
(B) Progesterone
(C) Human Chorionic Gonadotropin (HCG)
(D) Corticotropic Hormone
24. Followings are some opioid analgesics:
(P) Morphine (Q) Pethidine
(R) Pentazocine (S) Fentanyl
Choose the correct order of respiratory depressant propensity of these agents.
(A) P > Q > R > S
(B) Q > P > R > S
(C) R > P > Q > S
(D) S > P > Q > R
25. Corticosteroids are administered to treat some of the given disease states:
(P) Peptic ulcer (Q) Bronchial asthma
(R) Nephrotic syndrome (S) Myasthenia gravis
Choose the correct statement about the use of corticosteroids for the treatment of these diseases.
(A) P, Q and S are treated while R is NOT
(B) P, R and S are treated while Q is NOT
(C) Q, R and S are treated while P is NOT
(D) P, Q and R are treated while S is NOT
26. Which one of the following statements is FALSE for fluoroquinolones?
(A) These are highly effective by oral and parenteral routes
(B) These are relatively more susceptible to development resistance
(C) These are effective against those bacteria that are resistant to p-lactam and aminoglycoside antibiotics
(D) These are bactericidal with broad spectrum of activity
27. Increased serum levels of which one of the following may be associated with decreased risk of atherosclerosis?
(A) VLDL
(B) LDL
(C) HDL
(D) Total Cholesterol
28. Metformin causes the following actions EXCEPT for the one. Identify that:
(A) Reduces hepatic neoglucogenesis
(B) Increases glucose uptake in skeletal muscles
(C) Enhances sensitivity to insulin
(D) Increases HbAlc by 1% to 2%
29. Misoprostol has a cytoprotective action to gastro intestinal mucosa because of one of the following action. Identify that.
(A) It enhances secretion of mucus and bicarbonate ion
(B) It neutralizes hydrochloric acid in stomach
(C) It antagonizes nonsteroidal anti-inflammatory drugs
(D) It is bactericidal to H. pylori
30. Which of the following drugs can precipitate bronchial asthma?
(P) Indomethacin
(Q) Codeine phosphate
(R) Rabeprazole
(S) Theophylline
Choose the correct option.
(A) P and R can do that
(B) P and Q can do that
(C) R and S can do that
(D) S and Q can do that
31. Which one of the following alkaloids is derived from Lysine?
(A) Emetine
(B) Chelidonin
(C) Lobeline
(D) Stachydrine
32. Histologically the barks of Cinnamomum cassia and Cinnamomum zeylanicum differ in one of the following features. Identify that.
(A) Sclerieds
(B) Pholem Fibers
(C) Pericyclic Fibres
(D) Cortex
33. The following characteristic properties are given in context of saponins:
(P) Saponins give precipitate by shaking with water.
(Q) Saponins are diterpenes and given foam on shaking with water.
(R) Saponins are triterpenoidal compounds and cause haemolysis of erythrocytes.
(S) They are steroidal or triterpenoidal compounds with tendency to reduce surface tension of water.
Choose the correct option.
(A) P is true; Q is true: R is true: S is true
(B) P is false; Q is true: R is false: S is true
(C) P is false; Q is true; R is true; S is true
(D) P is false; Q is false; R is true; S is true
34. Read the given statements about the constituents of Shellac:
(P) Shellolic acid, a major component of alicyclic fraction is responsible for colour.
(Q) Shellolic acid, a major component of aromatic fraction is responsible for colour.
(R) Shellolic acid is a major component of aliphatic fraction and laccaic acid is a component of aromatic fraction.
(S) Aliphatic components are shellolic acid which is alicyclic and aleuratic acid which is acyclic, while laccaic acid is aromatic colouring principle.
What is the correct combination of options?
(A) P is true; Q is true; R is true; S is true
(B) P is false; Q is false; R is false; S is true
(C) P is false; Q is false; R is true; S is true
(D) P is true; Q is false; R is false; S is true
35. Major component of Cymbopogon citrates is citral which is utilized commercially for the followings;
(P) Synthesis of Vitamin A directly from citral
(Q) Synthesis of Vitamin A by first converting to T-ionone
(R) Synthesis of Vitamin A by first converting to T-ionone followed by conversion to a-ionone which is very important intermediate for cartenoid synthesis
(S) Synthesis of Vitamin A by first conversion of citral to T-ionone followed by conversion to P-ionone which is an important intermediate for carotenoid synthesis
Which is the correct combination of options?
(A) P is true; Q is true; R is true; S is true
(B) P is false; Q is true; R is false; S is true
(C) P is false; Q is false; R is true; S is true
(D) P is false; Q is false; R is false; S is false
36. Which one of the following constituents is reported to have anti-hepatotoxic activity?
(A) Podophyllotoxin
(B) Andrographoloid
(C) Linalool
(D) Safranal
37. Geranial and Neral are the monoterpene aldehyde constituents to volatile oil. Read the following statements about them:
(P) Geranial and Neral are both optical isomers
(Q) Geranial and Neral are both geometric isomers
(R) Geranial has Z configuration and Neral has E configuration
(S) Geranial has E configuration and Neral has Z configuration
Choose the correct combination of answers for them.
(A) P is true; Q is true; R is true; S is true
(B) P is false; Q is true; R is false; S is false
(C) P is true; Q is false; R is true; S is true
(D) P is false; Q is true; R is false; S is false
38. All of the followings applicable to Lignans are correct statements except for one. Identify the INCORRECT statement.
(A) Lignans are formed by the dimerization of the phenylpropane moiety
(B) Podophyllotoxin can be termed phytochemically as a lignan
(C) Lignans can be formed by cyclization of phenylpropane nucleus
(D) Lignans are the secondary metabolites formed from the Shikimic acid pathway
39. Naringin, obtained from orange peel, can be named as one of the followings. Identify the correct name.
(A) 5, 4’-Dihydroxy-7 rhamnoglucoside of flavanone
(B) 5, 4’-Dihydroxy-7-glucoside of flavanone
(C) 5,3’4’-Trihydroxy-7-rhamnoglucoside of flavone
(D) 5,3’,4’-Trihydroxy-7-glucoside of flavones
40. Rhizomes of Zingiber officinale contain some sesquiterpene hydrocarbons. Some hydrocarbons are given below.
(P) 3-Bisabolene (Q) Gingerone A
(R) Gingerol (S) Zingiberene
Identify the correct pair of constituents present in the rhizomes.
(A) P and S
(B) P and Q
(C) Q and S
(D) Q and R
41. Listed below are the chemical tests used to identify some groups of phytoconstituents. Identify the test for the detection of the purine alkaloids.
(A) Keller-Killanit Test
(B) Murexide Test
(C) Shinoda Test
(D) Vitali-Morin Test
42. Given below are four statements in context of Hecognein:
(P) It is a saponin
(Q) It is useful for the semi-synthesis of steroidal drugs
(R) It is not a glycoalkaloid
(S) It is obtained from Dioscorea tubers
Choose the correct combination of statements.
(A) P, Q and R are correct while S is incorrect
(B) P, Q and S are correct while R is incorrect
(C) Q, R and are correct while P, S are incorrect
(D) All are correct statements
43. Atropine biosynthesis involves a pair of precursors. Identify the correct pair:
(A) Ornithine and Phenylalanine
(B) Tyrosine and Tryptophan
(C) Tryptophan and Dopamine
(D) Tyrosine and Dopamine
44. Study the following statements:
(P) Lutein and zeaxanthin are flavonoids
(Q) Lutein and zeaxanthin are xanthophylls
(R) Lutein and zeaxanthin are required to control age-related macular degeneration
(S) Lutein is a flavonoid while zeaxanthin is its glycoside
Choose the correct answer:
(A) P is correct while Q, R and S are incorrect
(B) Q and R are correct while P and S are incorrect
(C) Statement P is the only correct statement
(D) Statement S is the only correct statement
45. Listed below are some phytoconstituents.
(P) Galactomannan (Q) Glucomannan
(R) Barbaloin (S) Phyllanthin
Identify the constituent(s) present in Aloe vera.
(A) Only P
(B) Q and R
(C) Only S
(D) P and S
46. Choose the correct answer for the binomial nomenclature of fruits of star-anise:
(A) Pimpinella
(B) Illicium verum
(C) Anisatum
(D) Religiosum
47. Given herewith are two statements:
(P) Digitoxin is a secondary glycoside from Digitalis purpurea
(Q) Digitoxin is a partially hydrolysed glycoside of Purpurea glycoside
Determine the correctness of the above statements.
(A) Both P and Q are true
(B) P is true but Q is false
(C) Both P and Q are false
(D) P is false but Q is true
48. Peruvoside is natural obtained from one of the following plants. Identify the correct name:
(A) Dioscorea
(B) Ginseng
(C) Liquorice
(D) Thevetia
49. One of the following is NOT required for the initiation and maintenance of plant tissue culture. Identify that.
(A) Sucrose
(B) Kinetin
(C) Auxin
(D) Absicic acid
50. Study the relationship between the given two statements:
(P) Capsanthin is a red coloured principle from Capscicum annum
(Q) Capsanthin is a vanillylamide of isodecenoic acid
Choose the correct answer:
(A) Both P and Q are correct
(B) Both P and Q are incorrect
(C) P is correct but Q is incorrect
(D) P is incorrect but Q is correct
51. For the equation PV = nRT to hold true for a gas, all of the following conditions are necessary EXCEPT for ONE. Identify that.
(A) The molecules of gas must be of negligible volume
(B) Collisions between molecules must be perfectly elastic
(C) The velocities of all molecules must be equal
(D) The gas must not be decomposing
52. Atracurium besylate, a neuromuscular blocking agent, is metabolized through one of the following reactions. Identify that:
(A) Hoffman
(B) Hoffman rearrangement
(C) Michael addition
(D) Claisen condensation
53. Identify the metabolic of prontosil responsible for its antibacterial activity:
(A) Sulphacetamide
(B) Sulphanilamide
(C) p-Amino benzoic acid
(D) Probenecid
54. The central bicyclic ring in penicillin is named as one of the following. Find the correct same.
(A) 1-Thia-4-azabicylo [3, 2, 1] heptanes
(B) 4-Thia-1-azabicylo [3, 2, 0] heptanes
(C) 4-Thia-1-azabicylo [3, 2] heptanes
(D) 1-Thia-4-azabicylo [1, 2, 3] heptanes
55. Both the CMR and PMR spectra of an unknown compound show four absorption peaks each. Identify the unknown compound.
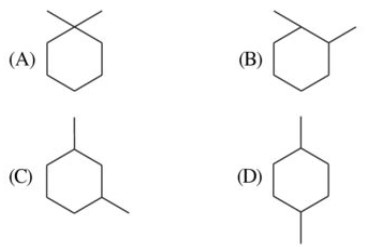
56. Out of the four given compounds choose the one which is aromatic?

57. Quantification of minute quantity of a drug from a complex matrix, without prior separation can be done using one of the following techniques. Identify that.
(A) Coulometry
(B) Potentiometry
(C) Fluorescence spectroscopy
(D) Radioimmunoassay
58. Which one of the following fragmentation pathways involves a double bond and a γ-hydrogen in mass spectrometry?
(A) α-Fission
(B) α1-Fission
(C) Mc-Lafferty rearrangement
(D) Retro-Diel’s Alder rearrangement
59. Read the following statements carefully about non-aqueous titrations:
(P) Acetate ion is the strongest base capable of existence in acetic acid
(Q) Mixtures of bases of different strengths can be analyzed by selecting a differentiating solvent for the bases.
(R) Acetic acid acts as a leveling solvent for various acids like perchloric and hydrochloric acids.
(S) Mixtures of bases of different strengths can be analyzed by selecting a leveling solvent for the bases.
Choose the correct answer.
(A) P and Q are true and R and S are false
(B) P and S are true and R and Q are false
(C) R and Q are true and P and S are false
(D) R and S are true and P and Q are false
60. Read the following statements carefully about Volhard’s method:
(P) In Volhard’s titration, silver ions are titrated with thiocyanates in acidic solution
(Q) Ferric ions act as indicator in Volhard’s method, yielding reddish brown ferric thiocyanate
(R) Volhard’s method is used to determine halides
(S) Volhard’s method is direct titration
Choose the correct set of answers.
(A) P, Q and R are true and S is false
(B) Q, R and S are true and P is false
(C) R, S and P true and Q I false
(D) P, Q, R and S all are true
61. Identify the group of enzymes that utilizes NADP or NAD as coenzymes and catalyzes biochemical reactions by the transfer of electrons from one molecule to another.
(A) Isomerases
(B) Oxidoreductases
(C) Transferases
(D) Ligases
62. Glucose is the only source of energy for one of the following. Identify that :
(A) Cardiac cells
(B) Nephrons
(C) RBCs
(D) Thrombocytes
63. Determine the correctness or otherwise of the following Assertion [A] and Reason [R]:
Assertion [A]: Halogens are unusual in their effect on electrophilic aromatic substitution; they are deactivating yet ortho-, para-directing.
Reason [R]: In electrophilic aromatic substitution reactions, reactivity is controlled by stronger inductive effect while orientation is controlled by the stronger hyperconjugation effect.
Choose the correct statement.
(A) [A] is true but [R] is false
(B) Both [A] and [R] are true and [R] is the correct reason for [A]
(C) Both [A] and [R] are false
(D) Both [A] and [R] are true but [R] is NOT the correct reason for [A]
64. Given are the four statements about dehydration of alcohols to given alkenes:
(P) Ease of dehydration of alcohols take place in the order 3° > 2° > 1°.
(Q) Dehydration is acid catalyzed.
(R) Orientation of the alkene formed is strongly Saytzeff.
(S) Dehydration is irreversible.
Choose the correct combination of statements.
(A) P and Q are correct while R and S are not
(B) P, Q and R all three are correct but S is not
(C) P, Q, R and S all are correct
(D) P, Q and S all three are correct but R is not
65. Choose the correct statement regarding the synthesis of phenyl para-propyl ether:
(A) Phenyl para-propyl ether is prepared from n-propyl bromide and sodium phenoxide
(B) Phenyl para-propyl ether is prepared from bromo-benzene and sodium para-propoxide
(C) Phenyl n-propyl ether can be prepared by either of the two methods
(D) Both (A) and (B) are not the correct methods for the synthesis of phenyl para-propyl ether
66. Read the following statements about SN1 reactions:
(P) The proceed with complete inversion (Walden inversion)
(Q) They proceed with racemizationplus some net inversion.
(R) They are characterized by rearrangements.
(S) They are characterized by the reactivity sequence, CH3>1°>2°>3°
Choose the correct combination.
(A) P and Q are true while R and S are false
(B) P and R are true while S and Q are false
(C) Q and R are true while P and S are false
(D) R and S are true while P and Q are false
67. Read the following statements carefully:
(P) Pyrrole and thiophene undergo electrophilic aromatic substitution reactions much faster than benzene
(Q) Pyrrole and thiophene undergo Diels Alder addition reaction very fast
(R) Pyrrole and thiophene undergo nucleophilic aromatic substitution reaction faster than benzene
(S) Pyrrole is a pie excessive system while thiophene is a pie deficient system.
Choose the correct combination of statements.
(A) Q only is true while P, R and S are false
(B) R and S are true while P and Q are false
(C) P and R are true while Q and S are false
(D) P only is true while Q, R and S are false
68. Among the followings which one is not only a non-reducing sugar but also does not exhibit mutarotation?
(A) Glucose
(B) Maltose
(C) Lactose
(D) Sucrose
69. Choose the most basic heterocyclic compound among the following.
(A) Pyridine
(B) Imidazole
(C) Pyrrole
(D) Pyrrolidine
70. Following are some drug derivatives used to increase/decrease the water solubility of the parent drugs:
(P) Rolitetracycline
(Q) Erythromycin lactobionate
(R) Chloramphenicol succinate
(S) Erythromycin stearate
Choose the correct combination of statements.
(A) Q and R are used to increase water solubility while P and S are used to decrease it
(B) P, Q and R are used to increase water solubility while P is used to decrease it
(C) Q, S and R are used to increase water solubility while P is used to decrease it
(D) Q and S are used to increase water solubility while P and R are used to decrease it
71. Study the following statements on prevention of crystalluria. By the given approaches crystalluria can be prevented.
(P) By co-administration of sulfadiazine, sulfamerazine and sulfamethazine
(Q) By increasing the pH of urine
(R) By co-administration of sulphanilamide, sulphamethoxazole and folic acid
(S) By administration of co-trimoxazole
Choose the correct combination of statements.
(A) P and Q are correct
(B) R and S are correct
(C) P and R are correct
(D) Q and R are correct
72. Progesterone is obtained from diosgenin through the following sequence of chemical reactions:
(P) Acetylation, CrO3 (oxidation), Acetolysis, H2/Pd, Hydrolysis and Oppenauer oxidation
(Q) Oppenauer oxidation, Acetylation, CrO3 (oxidation), Acetolysis, H2/Pd and Hydrolysis
(R) CrO3 (oxidation), Acetolysis, Acetylation, Oppenauer oxidation, Hydrolysis and H2/Pd
(S) Acetylation, H2/Pd, Hydrolysis, CrO3 (oxidation), Oppenauer oxidation and Acetolysis
Choose the correct sequence of reactions.
(A) P
(B) Q
(C) R
(D) S
73. Following statements are given for local anaesthetic drug lidocaine:
(P) It contains a xylidine moiety-gpatindia.com
(Q) It can be used as antiarrhythmic agent on oral administration.
(R) When administered along with adrenaline its toxicity is reduced and its effect is prolonged.
(S) Chemically it is 2-diethylamino-2’,6’-dimethyl-phenyl acetamide
Choose the correct combination of statements.
(A) P, Q and S
(B) P, Q and R
(C) P, R and S
(D) Q, R and S
74. One of the following ring systems can be used as the bioisosteric replacement for benzene ring in drug design:
(P) Thiophene (Q) Cyclohexa-1,3-diene
(R) Pyrrolidine (S) Imidazoline
Identify the correct answer
(A) P
(B) Q
(C) R
(D) S
75. Some of the following statements describe the properties of Dropping Mercury Electrode (DME) correctly:
(P) Constant renewal of electrode surface eliminates poisoning effects.
(Q) Mercury makes many metal ions easily reducible.
(R) Mercury has large hydrogen over-voltage.
(S) The electrode can get oxidized with ease.
Identify the correct combination.
(A) All statements P, Q, R and S are correct
(B) Statements P, Q and R only are correct
(C) Statements P, R and S only are correct
(D) Statements P, Q and S only are correct
76. Penicillin ring system is derived from two of the following amino acids:
(P) Alanine and methionine
(Q) Cysteine and valine
(R) Glycine and cysteine
(S) Methionine and leucine
Choose the correct pair.
(A) P
(B) Q
(C) R
(D) S
77. For the management of which disease the given drug tacrine is used ? Identify.
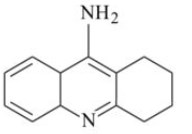
(A) Glaucoma
(B) Antidote for acticholinesterase poisoning
(C) As an insecticide
(D) Alzheimers disease
78. Low dose aspirin acts as anti-platelet aggregating agent by which one of the following mechanisms? Find the correct answer.
(A) It acts as a suicide substrate for COX-1 enzyme present in platelets
(B) It acts as a transition state analog for COX-2 enzyme present in the platelets
(C) It acts as a reversible inhibitor of lipoxigenase present in the platelets
(D) It acts as an affinity label of oxidoreductases present in the platelets
79. Some statements are given for clavulanic acid, sulbactam and tazobactam:
(P) All three lack the 6-acylamino side chain
(Q) All are potent inhibitors of the enzyme p-lactamase
(R) All the prodrugs of penicillin
(S) All have weak antibacterial activity
Choose the correct combination of statements.
(A) P, Q and R are true while S is false
(B) Q, R and S are true while P is false
(C) P, R and S are true while Q is false
(D) P, Q and S are true while R is false
80. Electrophilic aromatic substitution reactions in indole give one of the following products preferably. Identify that.
(A) 3-Substituted indole
(B) 2-Substituted indole
(C) 5-Substituted Indole
(D) 6-Substituted indole
81. Which one of the following species is an intermediate in the reaction shown below?
2CH3CH2CHO…..NaOH……..CH3CH2CH(OH).CH(CH3).CHO
(A) +CH2CH2CHO
(B) −CH2CH2CHO
(C) CH3+CHCHO
(D) CH3−CHCHO
82. Which detector is used in gas chromatography for halogen containing compounds specifically?
(A) Katharometer
(B) Electron capture detector
(C) Flame ionization detector
(D) Thermal conductivity detector
83. Precessional frequency of a nucleus depends on the following:
(P) Quantum of externally applied magnetic field
(Q) Quantum of electron density present around the nucleus
(R) Frequency of applied electromagnetic radiations
(S) Electronegativity of the element
Choose the correct combination of statements.
(A) P & Q are true
(B) P & R are true
(C) Q & R are true
(D) P & S are true
84. Some statements are given about disodium edetate:
(P) Disodium edetate is a bidentate ligand
(Q) Disodium edetate is a complexing agent but not a chelating agent
(R) Disodium edetate can be used for the assay of lithium carbonate
(S) Disodium edetate can be used for the assay of zinc sulphate
Choose the correct answer.
(A) Q, R & S are true
(B) Q & S are true
(C) S only is true
(D) P, Q R & S all are true
85. Which one of the following amino acids is the most effective contributor of protein buffer?
(A) Alanine
(B) Glycine
(C) Histidine
(D) Arginine
86. Given are some statements about cycloalkanes:
(P) Bayer’s theory does not apply to four membered rings.
(Q) Cyclohexane and cyclodecane rings are not flat but are puckered.
(R) Chair from of cyclohexane experiences van der Waals strain due to flagpole interactions.
(S) Boat form of cyclohexane experiences both torsional and Van der Waals strain.
Choose the correct combination of statements.
(A) P, Q & R are true and S is false
(B) Q & S are true and P & R are false
(C) P, Q & S are true and R is false
(D) Q, R & S are true and P is false
87. Phenols are more acidic than alcohols. This is due to one the following reasons. Identify that.
(A) Alkoxide ions are better stabilized by the electron releasing alkyl groups
(B) Resonance stabilizes both phenols and phenoxide ions to the same extent
(C) Phenols are better stabilized than the phenoxide ions while reverse is true for alcohols and alkoxides
(D) Phenoxide ions are much better stabilized than the alkoxide ions
88. Study the following statements on alkylating agents as antineoplastics:
(P) They get converted to aziridinium ions and bind to 7th position-N ato of guanine of DNA base pairs
(Q) Nitrogen mustards and Sulfur mustards belong to this class of drugs
(R) They inhibit dihydrofolate reductase enzyme thereby inhibiting DNA synthesis
(S) They chelate electropositive atoms present in the DNA thereby inhibiting DNA uncoiling
Choose the correct combination of statements.
(A) P and Q are correct
(B) R and S are correct
(C) P and S are correct
(D) Q and R are correct
89. Study the following statements about the stereochemistry of steroidal aglycones in cardiac glycosides:
(P) Rings A-B and C-D are cis fused while B-C is trans fused.
(Q) Rings A-B and C-D are trans fused while B-C is cis fused.
(R) Rings A-B are trans fused while B-C and C-D are els fused.
(S) Rings A-B are cis fused while B-C and C-D are trans fused.
Choose the correct statement.
(A) P is true while Q, R and S are false
(B) Q is true while P, R and S are false
(C) R is true while P, Q and S are false
(D) S is true while P, R and Q are false
90. Following are some statements about Captopril:
(P) It is a prototype molecule in the design of ACE inhibitors
(Q) It contains a sulphonyl group in its structure
(R) It has a proline moiety in its structure
(S) It has an ester linkage
Choose the correct combination of statements.
(A) P & Q are true while R & S are false
(B) Q & R are true while P & S are false
(C) P & R are true while Q & S are false
(D) R & S are true while P & Q are false
91. Cetirizine as an anthistaminic agent has a low sedative potential due to one of the following reasons. Identify that.
(A) It has a chiral center
(B) It has high log P value
(C) It has high polarity
(D) It has low molecular weight
92. There are some criteria which an ideal antacid should fulfill. Some of the criteria are given below:
(P) The antacid should be absorbable orally and should buffer in the pH range of 4-6
(Q) The antacid should exert its effect rapidly and should not cause a large evolution of gas
(R) The antacid should not be a laxative or should not cause constipation
(S) The antacid should react with the gastric acid and should inhibit pepsin.
Choose the correct combination of criteria for an ideal antacid.
(A) P, Q & R
(B) Q, R & S
(C) Q & R
(D) R & S
93. Titanium dioxide is used in sun screen products as a topical protective. The topical protective effect of titanium dioxide is arising due to one of the following properties. Identify that.
(A) It has a high bulk density
(B) It has a high LTV absorptivity
(C) It has a low water solubility
(D) It has a high refractive index
94. Deferoxamine is used for the treatment of toxicity caused by one of the following ions:
Identify that.
(A) Arsenic
(B) Cyanide
(C) Iron
(D) Lead
95. Parachor and Molar refraction can be categorized under one of the following properties. Identify that.
(A) Additive properties
(B) Constitutive properties
(C) Colligative properties
(D) Additive and constitute property
96. Rast’s camphor method is used for determination of molecular weight of solutes which are soluble in molten camphor. The basic principle of the method is dependent on one of the following properties. Identify that.
(A) Elevation of freezing point of camphor by the solute
(B) Lowering of vapour pressure of camphor by the solute
(C) Lowering of freezing point of camphor by the solute
(D) Elevation of boiling point of camphor by the solute
97. In polarography, when the limiting current is achieved, one of the following processes takes place.
(A) The rate of electron transfer just matches the rate of mass transfer
(B) The rate of electron transfer is slower than the rate of mass transfer
(C) The rate of electron transfer becomes independent of the rate of mass transfer
(D) The rate of electron transfer far exceeds the rate of mass transfer
98. Starch-iodide paste/paper is used as an external indicator in one of the following titrations.
Identify that.
(A) Iodometric titration of copper sulphate suing sodium thiosulphate as titrant
(B) Iodimetric titration of ascorbic acid using iodine solution is titrant
(C) Diazotisation titration of sulphadiazine using sodium nitrite as titrant
(D) Potassium dichromate titration using sodium thiosulphate as titrant
99. For a dye to be used as metal indicator in complexometric titrations, some of the dye properties are listed below:
(P) The dye should have distinct colour than the dye-metal complex
(Q) The dye-metal complex should have a higher stability than the metal-chelate (titrant) complex
(R) The dye should be capable of complexing with the metal ions
Choose the correct combination of statements for the dye to be used as an indicator in complexometric titrations.
(A) P & Q are correct while R is not
(B) Q & R are correct while P is not
(C) P & R are correct while Q is not
(D) P, Q and R all are correct
100. In amperometry, rotating platinum electrode (RPE) is used as indicating electrode. It has certain advantages as well as disadvantages. Read the following statements about the use of rotation platinum electrode in amperometry:
(P) It causes large diffusion current due to rotation resulting in greater mass transfer
(Q) It causes greatly reduced residual current due to lack of condenser effect
(R) It has a low hydrogen over potential
Choose the correct combination of statements.
(A) P, Q & R are all advantages of using RPE in amperometry
(B) P & R are advantages of RPE while Q is a disadvantage
(C) Q & R are advantages of RPE while P is a disadvantage
(D) P & R are advantages of RPE while R is a disadvantage
101. What will be the approximate Tmax of a drug exhibiting Ka of 2r” 1 and K of 0.2 hr-1?
(A) 1.2 hr
(B) 2.4 hr
(C) 4.8 hr
(D) 2.0 hr
102. There are some statements related to the protein binding of drugs as given below:
(P) Protein binding decreases the free drug concentration in the system
(Q) Protein binding to plasma albumin is an irreversible process.
(R) Drugs with a low lipophilicity have a high degree of protein binding.
(S) Protein binding of one drug can be affected by the presence of other drug.
Choose the correct combination of statements.
(A) P & Q are true while R & S are false
(B) Q & R are true while P & S are false
(C) R & S are true while P & Q are false
(D) P & S are true while Q & R are false
103. Based on Henderson-Hasselbalch equation, at what pH value a weak acid would be 99.9% ionized?
(A) At pH equivalent to pKa + 3
(B) At pH equivalent to pKa – 3
(C) At pH equivalent to pKa – 1
(D) At pH equivalent to pKa + 1
104. Some statements about crystals are given below:
(P) The crystal lattice is constructed from repeating units called unit cells
(Q) The external appearance of a crystal is described by crystal habits, such as needles, prisms, rosettes etc.
(R) Polymorphism is the ability of a compound to crystallize as more than one distinct crystalline species with different internal lattice.
(S) Hydrates are always more soluble than anhydrous form of the same drug.
Choose the corrected combination of statement about crystals.
(A) Statement P, Q and S are correct but R is wrong
(B) Statement P, Q and R are correct but S is wrong
(C) Statement Q, R and S are correct but P is wrong
(D) Statement R, S and P are correct but Q is wrong
105. Which one of the following is NOT used in preparation of baby powders
(A) Stearic acid
(B) Boric acid
(C) Kaolin
(D) Calcium carbonate
106. According to Kozeny Carmen equation a 10% change in porosity can produce:
(A) Two fold change in viscosity
(B) Five fold change in viscosity
(C) Three fold change in viscosity
(D) None of the above
107. Speed disk atomizer rotates at a speed of:
(A) 3000 – 5000 revolutions per min
(B) 3000 – 50000 revolutions per min
(C) 300 – 50000 revolutions per min
(D) 300 – 5000 revolutions per min
108. The Gold coating on a USP Dissolution apparatus-I basket should be:
(A) Not more than 2.5 in thickness
(B) Not more than 0.001 mm in thickness
(C) Not more than 0.025 p. in thickness
(D) Not more than 0.1 mm in thickness
109. Containers used for aerosols should withstand a pressure of:
(A) 130-150 Psig at 130 °F
(B) 140-180 Psig at 130 °F
(C) 140-170 Psig at 120 °F
(D) 120-140 Psig at 120 °F
110. Study the following two statements:
(X) If the gas is cooled below its critical temperature, less pressure is required to liquefy it.
(Y) At critical temperature and critical pressure, the liquid will have highest vapor pressure.
Choose the correct combination of statements.
(A) Both X and Y are correct
(B) X is incorrect and Y is correct
(C) X is correct and Y is incorrect
(D) Both X and Y are incorrect
111. Determine the correctness or otherwise of the following Assertion : [A] and the Reason [R]:
Assertion [A]: For an API of approximately same particle size, the angle of repose will increase with departure from spherical shape.
Reason [R]: Angle of repose is a function of surface roughness and particle size. With constant particle size, increase in roughness increases angle of repose.
(A) Although [A] is true but [R] is false
(B) Both [A] and [R] are false
(C) Both [A] and [R] are true and [R] is the correct reason for [A]
(D) Both [A] and [R] are true but [R] is NOT the correct reason for [A]
112. Study the following two statements:
(X) When used as granulating agent PEG 6000 improves dissolution rate of the dosage form as it forms a complex with a better solubility.
(Y) Sodium CMC when used as a binder affects dissolution rate of the dosage form as it is converted to less soluble acid form at low pH of the gastric fluid:
Choose the correct answer.
(A) Both X and Y are correct
(B) X is incorrect and Y is correct
(C) X is correct and Y is incorrect
(D) Both X and Y are incorrect
113. Study the following statements about Gram staining:
(P) Gram positive bacteria are stained deep violet and Gram negative bacteria are stained red.
(Q) Gram positive bacteria are stained red and Gram negative bacteria are stained deep violet.
(R) The sequence of addition of staining reagents is crystal violet, iodine solution, alcohol and safranin.
(S) In Gram positive bacteria the purple color developed during staining is lost during alcohol treatment. The cells later take up the safranin and stain red.
Choose the correct combination of statements.
(A) P, Q, R & S all are false
(B) P & Q are false and R & S are true
(C) P & S are false and Q & R are true
(D) P & R are false and Q & S are true
114. Choose the correct formula for the calculation of the retail price of a formulation, given by the Govt. of India..
(A) R.P. = (M.C. + E.D. + P.M. + P.C.) × (1 + MAPE/100)+ C.C.
(B) R.P. = (M.C. + C.C. + P.M. + P.C.) × (1 + MAPE/100)+ E.D.
(C) R.P. = (M.C. + C.C. + E.D. + P.C.) × (1 + MAPE/100)+ P.M.
(D) R.P. = (M.C. + C.C. + P.M. + E.D.) × (1 + MAPE/100)+ P.C.
115. Determine the correctness or otherwise of the following Assertion [A] and the reason [R]
Assertion [A] : In arsenic poisoning, dimercaprol, injected intramuscularly acts as antidote by metal complexation.
Reason [R] : EDTA acts as an antidote in lead poisoning, by solubilizing the toxic metal ions from the tissues.
(A) Although [A] is true but [R] is false
(B) Both [A] and [R] are false
(C) Both [A] and [R] are true and [R] is the correct reason for [A]
(D) Both [A] and [R] are true but [R] is NOT the correct reason for [A]
116. Determine the correctness or otherwise of the following Assertion [A] and the Reasons [R & S]:
Assertion [A] : Butylated hydroxytoluene is added as one of the ingredients in the lipstick formulation.
Reason [R] : It is a good solvent for the wax – oil mixtures and coloring pigments present in the lipstick.
Reason [S] : It is a antioxidant and prevents rancidity on storage.
(A) [A] is true, and [R] and [S] are true and correct reason for [A]
(B) [A], [R] and [S] are all false
(C) [A] is true, [S] is false, and [R] is the correct reason for [A]
(D) [A] is true, [R] is false, and [S] is the correct reason for [A]
117. Which one of the following statements is FALSE about Interferons?
(A) Interferons are cellular glycoproteins produced by virus infected cell
(B) Inteferons have not effect an extracellular virus
(C) Inteferons are virus specific agents that can interfere either with DNA or RNA virus
(D) They are produced as potent broad spectrum antiviral agents
118. In relation to sodium chloride and water mixture, read the following statements :
(P) Mixture is eutectic in nature
(Q) It has eutectic point -21.2°C
(R) The composition of eutectic is 25.3% by Mass
(S) The mixture is a true eutectoid and may exist as peritectic also. Which of the set of statements is correct?
(A) P & Q
(B) Q, R & S
(C) P, Q & S
(D) P, R & S
119. In relation to sterilization, what is the meaning of D300F – 2 minutes?
(A) Death of all microorganisms in 2 minutes
(B) Death of 300 microorganisms in 2 minutes
(C) Death of all microorganisms in 2 minutes at 300°F
(D) Death of 90% microorganisms in 2 minutes at 300°F
120. Choose the correct combination:
(i) Rod mill (p) Dried plant drug
(ii) Hammer mill (q) Thermolabile drug
(iii) Fluid energy mill (r) Paint
(A) i & q, ii & p, iii & r
(B) i & r, ii & q, iii & q
(C) i & q, ii & r, iii & p
(D) i & p, ii & q, iii & r
121. Which one of the following statements is NOT true for stainless steel 316?
(A) It is also called inox steel
(B) It contains 10.5-11% chromium
(C) Due to the presence of chromium it exhibits passivation phenomenon
(D) It is not affected by acids
122. Precise control of flow is obtained by which one of the following?
(A) Needle valve
(B) Butterfly valve
(C) Gate valve
(D) Globe valve
123. Heat sensitive materials like fruit juice are evaporated in which one of the following?
(A) Long tube vertical evaporator
(B) Calandria type evaporator
(C) Falling film type evaporator
(D) Forced circulation type evaporator
124. Which of the following conditions favour formation of large crystals?
(A) High degree of supersaturation
(B) Low nucleation rate
(C) High magma density
(D) Rapid cooling of magma
125. If M, L, T, Q and O are dimensional representations of mass, length, time, heat and temperature respectively, then what is the dimension of fluid thermal conductivity?
(A) Q/Mθ
(B) Q/TL2θ
(C) Q/TLθ
(D) M/LTθ
126. Which one of the following properties is characteristic of microemulsions?
(A) These are transparent systems with droplet size less than 1 MICRO METRE
(B) These are transparent systems with droplet size less than 10 MICRO METRE
(C) These are non-transparent systems with droplet size less than 1 MICRO METRE
(D) These are transparent systems with droplet size less than MICRO METRE
127. Which one of the following would be an offence in accordance with the provisions of the Drugs and Cosmetics Act, 1940?
(A) Packing of Paediatric oral drops in 30 ml pack
(B) Packing of Oxytocin injection in a single unit blister pack
(C) Packing of Schedule X drugs in 5 ml injection pack
(D) Packing of Aspirin tablets (75 mg) in 14 tablet strip pack
128. Which one of the following colours is NOT permitted to be used in drugs by the Drugs and Cosmetics Act, 1940?
(A) Chlorophyll
(B) Riboflavin
(C) Tartrazine
(D) Amaranth
129. At equal concentrations which one of the following mucilages will posses maximum viscosity?
(A) Maize starch
(B) Rice starch
(C) Wheat starch
(D) Potato starch
130. By which mechanism the microorganisms are killed by autoclaving?
(A) Coagulation of the cellular proteins of the microorganisms
(B) Alkylation of essential cellular metabolites of microorganisms
(C) Stopping reproduction of microorganism cells as a result of lethal mutations
(D) Oxidation of RNA of microorganisms
131. Manufacture and sale of some of the following drugs is prohibited in India:
(P) Fixed dose combination of atropine and antidiarrhoeals
(Q) Penicillin eye ointment
(R) Nimesulide paediatric drops
(S) Gatifloxacin tablets
Choose the drugs which are prohibited?
(A) P, Q & R
(B) Q, S & R
(C) R, S & P
(D) P, Q, R & S
132. Following are the phases of clinical trails:
(P) Human pharmacology
(Q) Therapeutic confirmatory trials
(R) Post marketing trials
(S) Therapeutic exploratory trials
Choose the correct order of phases of clinical trial.
(A) P, Q, R, S
(B) P, R, Q, S
(C) P, Q, S, R
(D) P, S, Q, R
133. The integrity of seals in case of vials and bottles is determined by some tests : Some of them are given below.
(P) Leaker’s test
(Q) Water hammer test
(R) Spark tester probe.
Choose the correct answer.
(A) P & Q
(B) Q & R
(C) P & R
(D) P, Q & R all
134. The integrity of seals in case of vials and bottles is determined by some tests : Some of them are given blow.
(P) Gram negative bacteria produce potent pyrogenic substances called endotoxins
(Q) Ethylene oxide mixed with carbon dioxide or fluorinated hydrocarbons is used in gas sterilization
(R) D value is the time (for heat or chemical exposure) or the dose (for radiation exposure) required for the microbial population to decline by one logarithmic unit
(S) Spores of Geobacillus stearothermophilus (Bacillus stearothermophilus) are used for sterility testing of moist heat sterilization process
Choose the correct answer :
(A) P, Q & R are correct but S is incorrect
(B) Q, R & S are correct but P is incorrect
(C) R, S & P are correct but Q is incorrect
(D) P, Q, R & S all are correct
135. Read the following statements:
(P) The surface area measurement using BET approach utilizes argon gas for adsorption
(Q) Full form of BET is Brunauer, Emmett and Teller
Choose the correct answer :
(A) P & Q are correct
(B) P is correct but Q is incorrect
(C) Q is correct but P is incorrect
(D) Both P & Q are incorrect
136. Based on the DLVO theory of force of interaction between colloidal particles, which one of the followings lead to attractive interaction between two particles?
(A) Solvation forces
(B) Electrostatic forces
(C) van der Waals forces
(D) Steric forces
137. Read the following statements with regard to viscosity of a polymer solution:
(P) Specific viscosity of a polymer solution is obtained as relative viscosity +1
(Q) Relative viscosity is the ratio of the viscosity of the solution to the viscosity of pure solvent
(R) Kinematic viscosity is defined as the viscosity of the liquid at a defined as the viscosity of the liquid at a definite temperature
(S) The unit for kinematic viscosity is poise or dyne sec cm2 Indicate the correct combination of statements.
(A) P & S are correct but Q & R are wrong
(B) Q & R are correct but P & S are wrong
(C) P & Q are correct but R & S are wrong
(D) R & S are correct but P & Q are wrong
138. Determine the correctness or otherwise of the following Assertion [A] and the Reason [R]
Assertion [A] : Salts having no ions in common with the slightly soluble electrolyte increase its solubility
Reason [R] : Such salts lower the activity coefficient of the slightly soluble electrolyte
(A) Both [A] and [R] are true and [R] is the correct reason for [A]
(B) Both [A] and [R] are false
(C) Although [a] is true but [R] is false
(D) Both [A] and [R] are true but [R] is NOT the correct reason for [A]
139. What negative adsorption would do?
(A) Decrease the surface free energy as well as the surface tension
(B) Increase the surface free energy as well as the surface tension
(C) Decrease the surface free energy but increase the surface tension
(D) Increase the surface free energy but decrease the surface tension
140. Read the following statements:
(P) At temperature below Kraft point, micelles will, not form
(Q) At Kraft point, solubility of surfactant equals CMC
(R) Kraft point increases with increasing chain length of hydrocarbon
(S) Kraft point is normally exhibited by non-ionic surfactants
Choose the correct combination of answers.
(A) P is correct but Q, R & S are wrong
(B) R & S are correct but P & Q are wrong
(C) P, Q & R are correct but S is wrong
(D) All are correct
141. Two statements are given regarding the uniformity of dispersion test (L. P.):
(P) It is evaluated using 6 tablets and 500 mL water
(Q) It involves measuring the dispersion time of each tablet
Choose the correct set of statements.
(A) P is correct while Q is incorrect
(B) P & Q both are correct
(C) P is incorrect while Q is correct
(D) Both P & Q are incorrect
142. Read the following statements:
(P) Caramelization occurs in acidic conditions
(Q) Caramel is optically inactive glucose
(R) Caramel is obtained by burning of glucose
(S) Caramel is obtained by degradation of fructose
Choose the right combination of statements.
(A) P & Q are true but R & S are false
(B) P & S are true but Q & R are false
(C) Q & R are true but P & S are false
(D) R & S are true but P & Q are false
143. Read the following statements regarding value added tax (VAT):
(P) It is an indirect tax
(Q) It is charged at the rate of 8%
(R) It is tax at source
(S) It is effective since April 2010
Choose the correct option.
(A) P & Q are true R & S are false
(B) R & S are true P & Q are false
(C) P & R are true Q & S are false
(D) Q & S are true P & R are false
144. Find the process by which the conversion of sulfasalazine to sulfapyidine and 5-amino salicylic acid takes place in the colon?
(A) Hydrolysis
(B) Deamination
(C) Acetylation
(D) Azoreduction
145. How much quantity (in grams) of sodium chloride is needed to make 30 ml of a 2% isotonic drug (sodium chloride equivalent 0.20) solution?
(A) 0.60
(B) 0.27
(C) 0.15
(D) 0.12
146. Read the following statements about lyophilization:
(P) Lyophilization cannot be done in final containers like multiple dose containers.
(Q) Lyophilized product needs special methods for reconstitution.
(R) Lyophilization causes protein denaturation in tissues.
(S) Lyophilization causes protein denaturation in tissues.
Choose the correct combination of statements.
(A) P is true and Q, R & S are false
(B) Q is true and P, R & S are false
(C) R is true and P, Q & S are false
(D) S is true and P, Q & R are false
147. In a pharmacokinetic model depicted in the following scheme, what is the half-life of the drug if the apparent volume of distribution of the drug is 25 L?
(A) 1.7 hr
(B) 2 hr
(C) 4 hr
(D) 3 hr
148. A sample of paracetamol tablets claims to contain 500 mg of paracetamol. But, on analysis by Govt. Analyst, it was found to contain 200 mg. As per Drugs and Cosmetics Act, 1940, this product would be categorized as what?
(A) Misbranded drug
(B) Adulterated drug
(C) Spurious drug
(D) Unethical drug
149. Use of which of the following artificial sweeteners is permitted in various dosage forms of Ayurveda, Siddha and Unani proprietary medicines?
(A) Sucralose
(B) Aspartame
(C) Saccharin
(D) All of them
150. What will be the maintenance dose of a sustained release 12 hour formulation of drug X exhibiting one compartment kinetics with a half-life of 6 hours, plasma concentration (steady state) 6 μg/ml, volume of distribution 30 L, and an oral bioavailability of 80%?
(A) 249.48 mg
(B) 225.48 mg
(C) 311.85 mg
(D) 281.85 mg
Latest Govt Job & Exam Updates: