| Go to all OUAT Previous Year Question Papers | 2022, 2021, 2020, 2019, 2018, 2017, 2016, 2015, 2014, 2013, 2012,2011, 2010, 2009, 2008, 2007 |
OUAT Previous Question Paper-2019
MENTAL APTITUDE
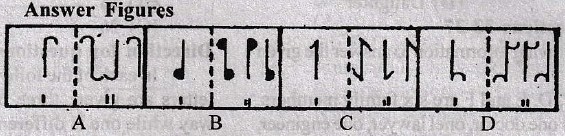
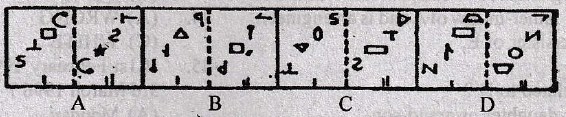
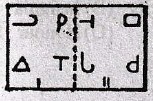
Directions for question 1-10:
In each of these questions, there are two separate figures. The figures on the left are Problems Figures (four figures and one question-marked space) and those on the right are Answer Figures indicated by letters A, B, C and D. A series is established if one of the four Answer Figures is placed at the “question-marked space”. Figures form a series if they change from left to right according to same rule. The letter of the Answer Figure which should be placed in the question-marked space is the answer. All the five figures, i.e., four Problem Figures and one Answer Figure placed in the question-marked space should be considered as forming the series.
1.

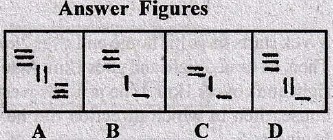
2.


3.
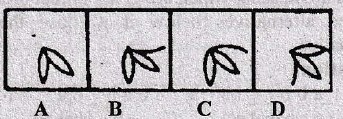
4. 
5. 

6. 

7. 

8. 

9. 

10. 

11. Vivek starts from his home and goes 2 km straight. Then, he turned right and goes 1 km. Again he turns right and covers 1km. If he is north-west from h is house, then in which direction did he go in the beginning?
(A) North
(B) South
(C) East
(D) West
Direction for questions 12-14
In each of the following letter series, some of the letters are missing which are given in that order as one of the alternatives below it. Choose the correct alternative.
12. _a b b _ _bb _ a _ b b a b _ b a
(A) bababa
(B) bbabbb
(C) ababaa
(D) aaaabb
13. b _ a c _ c c _ c b _ a b _ a c
(A) ababa
(B) bbaac
(C) abbbc
(D) aabba
14. _ c _ b d _ c b c d a _ a _ d b _ a
(A) adabcd
(B) cdbbca
(C) daabbc
(D) bdbcba
15. If Bangle is called Cassette, Cassette is called Table, Table is called Game, Game is called Cupboard and Cupboard is called Bangle, then which is played in the tape recorder?
(A) Table
(B) Bangle
(C) Cupboard
(D) Cassette
Direction for question 16-20
Each question is followed by two statements (1) and (2).
Indicate
(A) If statement (1) ALONE is sufficient, but Statement (2) alone is not sufficient.
(B) If statement (2) ALONE is sufficient, but Statement (1) alone is not sufficient.
(C) If EACH statement ALONE is sufficient
(D) If Statements (1) and (2) TOGETHER are NOT sufficient.
16. IS X > y?
(1) x = y + 2
(2) ![]()
17. Last year the average (arithmetic mean) salary of the 10 employees of Company X was Rs. 42800/-. What is the average salary of the same 10 employees this year?
(1) For 8 of the 10 employees, this year’s salary is 15 percent greater than last year’s salary.
(2) For 2 of the 10 employees, this year’s salary is the same as last year’s salary.
18. In a certain classroom, there are 80 books, of which 24 are fiction and 23 are written in English. How many of the fiction books are written in English?
(1) Of the fiction books, there are 6 more that are not written in English than are written in English.
(2) Of the books written in English, there are 5 more nonfiction books than fiction books.
19. If n is an integer, is n + 1 odd?]
(1) n + 2 is an even integer.
(2) n – 1 is an odd integer.
20. The hypotenuse of a right triangle is 10 cm. What is the perimeter, in centimeters, of the triangle?
(1) The area of the triangle is 25 sq.cm.
(2) The 2 legs of the triangle are of equal length
21. In a certain code ALMIRAH is written as BNPMWGO, which word would be written as DNRWLUA?
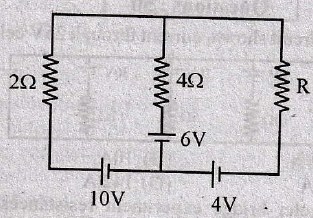
(A) COSGOLT
(B) TOGSOLC
(C) TOGCLOS
(D) CLOSGOT
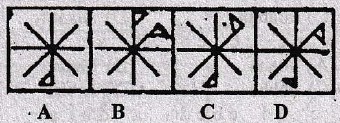
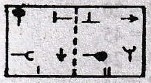
Direction for question 22-31
In each of the questions, a related pair of figures (unnumbered) is followed by four numbered pairs of figures. out of these four, three have relationship similar to that in the original pair. Only one pair of figures does not have similar relationship. Select the pair of figures which does NOT have a similar relationship to that in the unnumbered pair. Letter of that figures is your answer.
22. 
23. 

24. 
25. 
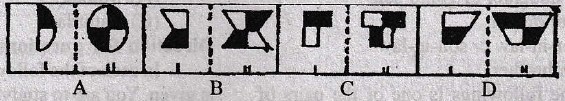
26. 

27. 

28. 

29. 

30. 

31. 

32. Pointing to a person a man said to a woman, “His mother is the only daughter of your father”. How was the woman related to the person?
(A) Aunt
(B) Mother
(C) Wife
(D) Daughter
Direction for question 33-37
Read the following information to answer the given question.
(i) A, B, C, D E and F are six family members.
(ii) There is one doctor, onel lawyer, one engineer, two students and one house wilfe.
(iii) There are two married couples in the family.
(iv) F, who is a doctor, is father of E.
(v) A is a student, and her husband is not a lawyer.
(vi) B is the grandmother of D and is a housewife.
(vii) C is the father-in-law of A and is an engineer.
(viii) D is the sister of E.
33. How is E relate to B?
(A) Grand-daughter or grand-son
(B) Daughter or son
(C) Daughter-in-law or son-in-law
(D) Sister or brother
34. Which of the followings is one of the pairs of married couples?
(A) FB
(B) FA
(C) CF
(D) FD
35. How many female members are there in the family?
(A) Three
(B) Two
(C) Three or four
(D) Two or Three
36. Who is the doctor?
(A) D
(B) E
(C) A’s husband
(D) C’s wife
37. Which of the following statements is definitely TRUE?
(A) C is the father of the student
(B) B is the mother of the student.
(C) D is the sister of the lawyer.
(D) F is the father of the lawyer.
Direction for questions 38-41
In each of the followings questions, one term in the number series if WRONG. Find out the wrong term.
38. 5, 27, 61, 122, 213, 340, 509
(A) 27
(B) 61
(C) 122
(D) 509
39. 46080, 3840, 384, 48, 24, 2, 1
(A) 384
(B) 48
(C) 24
(D) 2
40. 15, 16, 22, 29, 45, 70
(A) 16
(B) 22
(C) 45
(D) 70
41. 0, 2, 3, 5, 8, 10, 15, 18, 24, 26, 35
(A) 18
(B) 24
(C) 26
(D) 10
Direction for questions 42-44
In each of the following questions, four groups of letters are given; three of them are alike in a certain way while one is different. Choose the ODD one.
42.
(A) EBD
(B) IFH
(C) QNO
(D) YVX
43.
(A) ABDG
(B) IJLO
(C) MNPS
(D) RSUY
44.
(A) WRONG
(B) PRONE
(C) WHITE
(D) RIGHT
45. If 1st February 1996 is Wednesday, what day is 3rd March 1996?
(A) Monday
(B) Sunday
(C) Saturday
(D) Friday
Direction for questions 46-49
In each of the following questions, a pair of words is given. You are to study the relation existing between them and then find out from the given alternatives, the pair of words that bears the same relation between them and indicate that on the answer sheet.
46. Heart : Cardiology
(A) Brain : Psychology
(B) History : Histology
(C) Civics : Polity
(D) Fossils : Paleontology
47. Horse : Mare
(A) Duck : Geese
(B) Dog : Puppy
(C) Donkey : Pony
(D) Fox : Vixen
48. Flag : Nation
(A) Emblem : Prosperity
(B) Insignia : Rank
(C) Wealth : Prestige
(D) Honour : Status
49. Ornaments : Body
(A) Murals : Walls
(B) Painting : Canvas
(C) Light : Road
(D) Cleanliness : Hospital
50. If + stands for ÷, × stands for +, − stands for and stands ÷ for −, then which of the following equations is correct?
(A) 36 × 6 + 7 ÷ 2 – 6 = 20
(B) 36 ÷ 6 + 3 × 5 – 3 = 45
(C) 36 + 6 – 3 × 5 ÷ 3 = 24
(D) 36 – 6 + 3 × 5 ÷ 3 = 74
PHYSICS
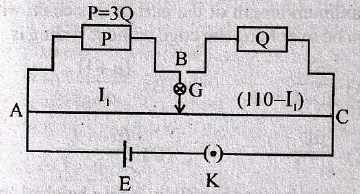
51. In the circuit shown, current through 25V cell is

(A) 7.2 A
(B) 10A
(C) 12A
(D) 14.2A
52. In a meter bridge experiment resistances are connected as shown in the figure. The balancing length is 55 cm. Now an unknown resistance x is connected ins series with P and the new balancing length is found to be 75 cm. The value of x is
(A) ![]()
(B) ![]()
(C) ![]()
(D) ![]()
53. Four persons are initially at the four corners of a square whose side is equal to d. Each person now moves with a uniform speed V in such a way that the first move directly towards the second, the second directly towards the third, the third directly towards the fourth and the fourth directly towards the first. The four persons meet after a time equals to
(A) d/V
(B) 2d/3V
(C) 2d/√3V
(D) d/√3V
54. In a stack of three polarizing sheets, the first and the third are crossed while the middle one has its axis at 45° to the axes of the other two. The fraction of intensity of an incident unpolarized beam of light that is transmitted by the stack is
(A) 1/2
(B) 1/3
(C) 1/4
(D) 1/8
55. When a beam of 10.6 eV photons of intensity 2.0 W/m2 falls on a metallic surface of area 1 × 10−4 m2 and work function 5.6 eV, 0.53% of incident photons eject photoelectrons. The number of photoelectrons emitted per second is
(A) 1.18 × 1016
(B) 6.25 × 1011
(C) 6.25 × 1013
(D) 6.25 × 1015
56. A uniformly charged thin spherical shell of radius R carries uniform surface charge density of σ. It is made of two hemispherical shells, held together by pressing them with force F. F is proportional to

(A) 
(B) 
(C) 
(D) ![]()
57. If mean life of a radioactive sample about___ of the substance disintegrates.
(A) 2/3
(B) 1/3
(C) 90%
(D) 50%
58. Refer to the figure, Potential difference between A and B is

(A) Zero
(B) 5 V
(C) 10 V
(D) 15 V
59. A conducting circular loop is placed in a uniform magnetic field, B = 0.025 T with its plane perpendicular to the loop. The radius of the loop is made to shrink at a constant rate of 1 mm S−1. The induced emf when the radius is 2 cm, is
(A) 2 π μ V
(B) π μ V
(C) ![]()
(D) 2μV
60. The charge on the capacitor in steady state in the circuit shown is

(A) 0.5 μ C
(B) 1 μ C
(C) 2 μ C
(D) 4 μ C
61. The relation between time t and distance x for a moving particle is t = αx2 + βx, where α and β are constants. If V is the velocity at distance x, then the retardation of the particle is
(A) 2 αV3
(B) 2 βV3
(C) 2 α βV3
(D) 2 β2V2
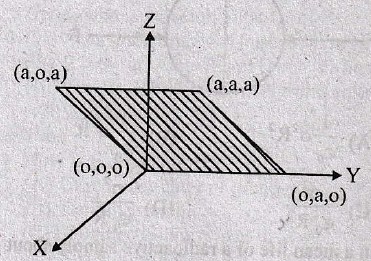
62. Consider an electric field, ![]() where E0 is a constant. The flux through the shaded area (as shown in figure) due to this field is
where E0 is a constant. The flux through the shaded area (as shown in figure) due to this field is
(A) 2 E0a2
(B) √2 E0a2
(C) E0a2
(D) E0a2/√2
63. An Alpha particle accelerated through V volts is fired towards a nucleus. Its distance of closest approach is r. If a proton accelerated through the same potential is fired towards the same nucleus, Its distance of closest approach will be
(A) r
(B) 2r
(C) r/2
(D) r/4
64. Determine the r.m.s value of a semicircular current wave which has a maximum value of a.
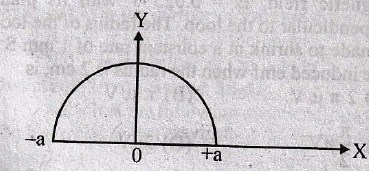
(A) 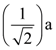
(B) ![]()
(C) ![]()
(D) 
65. For what value of R in the circuit as shown, current through 4Ω will be zero?
(A) 1Ω
(B) 2 Ω
(C) 3 Ω
(D) 4 Ω
66. A uniform chain of length L meters is lying over a table. If the coefficient of friction be μ, then the maximum length of the part of this chain which can be over hung the edge without sliding is
(A) ![]()
(B) ![]()
(C) ![]()
(D) ![]()
67. A ball is dropped vertically from a height d above the ground. It hits the ground and bounces up vertically to a height d/2. Neglecting subsequent motion and air resistance, its velocity V varies with height h above the ground, as
(A) 
(B) 
(C) 
(D) 
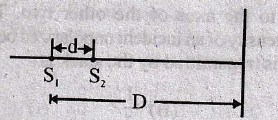
68. Two coherent sources S1 and S2 are separated by a small distance d as shown. The fringes obtained on the screen will be
(A) Points
(B) Straight lines
(C) Semi-circle
(D) Concentric circles
69. When only carrier is transmitted, antenna current observed is 8A. When it is modulated with 500 Hz sine wave antenna current becomes 9.6 A. The modulation is
(A) 80%
(B) 20%
(C) 94.26%
(D) 83.76%
70. An ideal gas is kept in a closed vessel having capacity 0.0083 m3 at a temperature of 300k and a pressure of 1.6 × 106m−2. The specific heat of an ideal gas at constant pressure is ![]() If 2.49 × 104 J amount of heat energy is supplied to the gas, then final pressure of the gas will be
If 2.49 × 104 J amount of heat energy is supplied to the gas, then final pressure of the gas will be
(A) 1.6 × 106 N.m−2
(B) 2 × 106 N.m−2
(C) 2.5 × 106 N.m−2
(D) 3.6 × 106 N.m−2
71. A composite slab made up of two different materials having thickness 2x and 8x, thermal conductivity 2k and 4k respectively and temperatures T2 and T1(T2 > T1). The rate of heat transfer through the slab, In a steady state is ![]() f with f equals to
f with f equals to
(A) 1
(B) 1/2
(C) 2/3
(D) 1/3
72. A neutron inelastically collide with a single inoised helium atom at rest with kinetic energy = 65 eV, making a scattering angle 90° with respect to the original direction. The allowed values of the energy of the neutron after the collision are
(A) 6.36 eV and 0.312 eV
(B) 17.84 eV and 16.32 eV
(C) 1.8 eV and 15.8 eV
(D) 16.328 eV and 6.36 eV
73. A ray of light is incident on a surface of glass slab at an angle 45°. If the lateral shift Produced per unit thickness is 1/√3 m, the angle of refraction produced is
(A) 
(B) 
(C) 
(D) 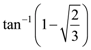
74. During charging a capacitor, the variation of potential V of the capacitor with time t is
(A) 
(B) 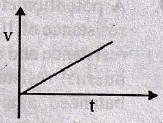
(C) 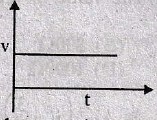
(D) 
75. A girl uses a simple pendulum of 1 m length to calculate the acceleration due to gravity g. She uses a stop watch with the least count of 1 sec for this and records 60 sec for 30 oscillations. For this observation, which of the following statements is TRUE?
(A) Percentage error in g is 2.5%
(B) Percentage error in g is 3%
(C) Percentage error in g is 3.3%
(D) Percentage error in g is 2%
76. The amplitude of a wave represented by displacement equation  will be
will be
(A) ![]()
(B) ![]()
(C) ![]()
(D) 
77. The wavelength of the first spectral line of the Balmer series of hydrogen atom is 5461 A. The wavelength of the second spectral line in the Balmer series of singly ionsied helium atom is
(A) 1215 A
(B) 1640 A
(C) 2430 A
(D) 1011.3 A
78. In electromagnetic waves travelling in vacuum ⇒
(1) The electric field ![]() is always perpendicular to the magnetic field
is always perpendicular to the magnetic field ![]()
(2) ![]() always gives the direction in which the waves travel
always gives the direction in which the waves travel
(3) The fields ![]() and
and ![]() vary sinusoidally
vary sinusoidally
(4) There is a phase difference between ![]() and
and ![]() is π/2 The correct statement(s) is (are)
is π/2 The correct statement(s) is (are)
(A) 1, 3
(B) 1, 3, 4
(C) 1, 2, 3, 4
(D) 1, 2, 3
79. A potentiometer wire of length 100 cm has a resistance of 10Ω. It is connected in series with a resistance and a cell of emf 2 V and of negligible internal resistance. A source of emf 10 mV is balanced against a length of 40 cm of the potentiometer wire. The value of the external resistance is
(A) 790Ω
(B) 890Ω
(C) 990Ω
(D) 1090Ω
80. A ray of light travelling in glass  is incident on a horizontal glass-air surface at the critical angle. If a thin layer of water
is incident on a horizontal glass-air surface at the critical angle. If a thin layer of water  is poured on the glass-air surface. The angle at which the ray emerges into air at the water-air surface is
is poured on the glass-air surface. The angle at which the ray emerges into air at the water-air surface is

(A) 0
(B) π/4
(C) π/3
(D) π/2
81. In the circuit below A and B represent two inputs and C represents the output.
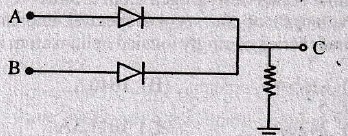
The circuit represents
(A) NOR gate
(B) AND gate
(C) NAND gate
(D) OR gate
82. In a common emitter configuration with suitable bias, it is given that RL is the load resistance and RBE is small signal dynamic resistance (input side). Then voltage gain, current gain and power gain are given respectively (β is current gain IB, IC, IE are respectively base, collector and emitter currents)
(A) 
(B) 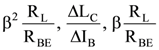
(C) 
(D) 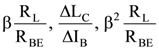
83. A particle of charge per unit mass α is released from origin with a velocity ![]() in a magnetic field.
in a magnetic field. 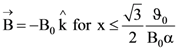 The x coordinate of the particle at time
The x coordinate of the particle at time  would be
would be
(A) 
(B) 
(C) 
(D) 
84. A particle of mass m is moving with a constant velocity along a line parallel to the positive direction of X-axis. The magnitude of its angular momentum with respect to the origin.
(A) is zero
(B) goes on increasing as x increases
(C) goes on decreasing as x increases
(D) remains constant for all positions of the particle
85. The angle of prism and refractive index of the material of the prism are A and ![]() The angle of minimum deviation of the prism is
The angle of minimum deviation of the prism is
(A) ![]()
(B) π – A
(C) ![]()
(D) π – 2A
86. In a certain mass spectrometer, an ion beam passes through a velocity filter consisting of mutually perpendicular fields ![]() The beam then enters a region of another magnetic field
The beam then enters a region of another magnetic field ![]() perpendicular to the beam. The radius of curvature of the resulting ion beam is proportional to
perpendicular to the beam. The radius of curvature of the resulting ion beam is proportional to
(A) Eʹ/B
(B) EB/Bʹ
(C) BBʹ/E
(D) E/BBʹ
87. In Young’s double slit experiment using monochromatic light of wavelength λ, the intensity of light at a point on the screen where path difference is λ, is k units. The intensity of light at a point where path difference is λ/3, is
(A) k/2
(B) k/3
(C) k/4
(D) 2k/3
88. Figure shows a thin rectangular copper plate with the centra of mass at origin O and side AB = 2BC = 2m. If a quarter part of the plate (shown as shaded) is removed, the centre of mass of the remaining plate would lie at
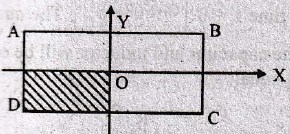
(A) ![]()
(B) ![]()
(C) ![]()
(D) ![]()
89. Two solid spheres of the same metal but of mass M and 8M fall simultaneously in a viscous fluid. If their terminal velocities are υ and nυ, then the value of n will be
(A) 16
(B) 8
(C) 4
(D) 2
90. A small satellite is in elliptical orbit around the earth as shown in the figure. L denotes the magnitude of its angular momentum and K denotes its kinetic energy. If 1 and 2 denotes two positions of the satellite, then

(A) L2 = L1, K2 = K
(B) L2 = L1, K2 > K1
(C) L2 > L1, K2 < K1
(D) L2 = L1, K2 < K1
91. The force F is given in terms of time t and displacement x by the equation F = A cos Bx + C sin Dt. The dimensional formula of D/B is
(A) [M0L0T0]
(B) [M0L0T−1]
(C) [M0L−1T0]
(D) [M0L1T−1]
92. Springs of spring constant K, 3K, 9K, 27K,…… ∞ are connected in series. Equivalent spring constant of the combination is
(A) 3K/2
(B) K/2
(C) 2K/3
(D) ∞
93. Different curves in the figure show the behaviour of gases.

(1) The curve I represents ideal gas behaviour.
(2) Curves II, III and IV also represent ideal gas behaviour at different temperatures T2, T3 and T4
(3) Curves II, III and IV represent behaviour of a real gas at different temperatures T2, T3 and T4
The correct statements are
(A) 1, 2, 4
(B) 2, 3, 4
(C) 1, 3, 4
(D) 1, 2, 3
94. Two bodies of masses m and 4m are attached with a string as shown in the figure below. The body of mass m ranging from a string of length I is executing oscillation of angular amplitude θ while the other body is at rest. The minimum coefficient of friction between the mass 4m and the horizontal surface should be

(A) 
(B) 
(C) 
(D) 
95. A rocket is moving at a speed of 200 ms−1 towards a stationary target. While moving it emits a wave of frequency 1000 Hz. Some of the sound reaching the target gets reflected back to the rocket as an echo. The frequency of the echo as detected by the rocket is (velocity of sound = 330 ms−1)
(A) 1000 Hz
(B) 1580 Hz
(C) 2504 Hz
(D) 4080 Hz
96. A convex lens has a focal length f. It is cut into two parts along the dotted line as shown in the figure. The focal length of each part will be

(A) f/2
(B) f
(C) 3f/2
(D) 2f
97. A steady current is set up in a cubic network composed of wires of equal resistance and length d as shown in the figure. What is the magnetic field at the centre P due to the cubic network?
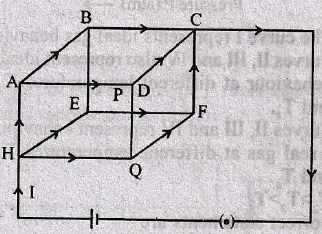
(A) ![]()
(B) ![]()
(C) 0
(D) ![]()
98. An electron is placed on X-axis where the electric potential depends on x as shown in the figure (the potential does not depend on y and z). What is the electric force on the electron?
(A) 4.0 × 10−18N
(B) 8.0 × 10−18N
(C) 3.2 × 10−16N
(D) 4.0 × 10−16N
99. The potential field depends on x and y-coordinates as V = x2 – y2. Corresponding electric field lines in X-Y plane are as
(A) 
(B) 
(C) 
(D) 
100. In the circuit shown in the figure, the switch S is closed at time t = 0 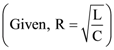 The current through the capacitor and inductor will be equal at a time t equals to
The current through the capacitor and inductor will be equal at a time t equals to
(A) RC
(B) RC In 2
(C) ![]()
(D) LR
CHEMISTRY
101. The coefficients of I−, IO3− and H+ in the redox reaction, I− + IO3− + H+ → I2 + H2O in the balanced, from respectively are
(A) 5, 1, 6
(B) 1, 5, 6
(C) 6, 1, 5
(D) 5, 6, 1
102. The solubility of alkali metal hydroxide is
(A) LiOH < KOH < NaOH < RbOH < CsOH
(B) LiOH < NaOH < KOH < RbOH < CsOH
(C) CsOH < RbOH < KOH < NaOH < LiOH
(D) None of these
103. The main reason that SiCl4 is easily hydrolysed as compared to CCI4 is that
(A) Si-Si bond is weaker
(B) SiCl4 can form hydrogen bonds
(C) SiCl4
(D) Si can extend its coordination number beyond four
104. The total number of π – bond electrons in the following structure is
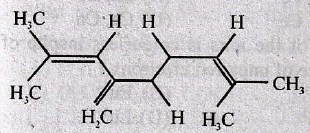
(A) 4
(B) 8
(C) 12
(D) 16
105. Acid catalysed hydxation of alkene is an example for
(A) free radical substitution
(B) nucleophilic substitution
(C) nucleophilic addition
(D) electropilic addition
106. Assertion (A) : CH4 does not react with Cl2 in dark. Reason (R) : Chlorination of CH4 takes place in sunlight.
(A) Both A and R are correct and R is correct explanation of A.
(B) Both A and R are correct but R is not correct explanation of A.
(C) A is correct but R is incorrect.
(D) Both A and R are incorrect.
107. Copper crystallizes in FCC lattice with a unit cell edge of 361 pm. The radius of copper atom is
(A) 181 pm
(B) 108 pm
(C) 128 pm
(D) 157 pm
108. Which of the following has the highest value of energy gap?
(A) Aluminium
(B) Silver
(C) Germanium
(D) Diamond
109. The molarity of H2SO4 solution, which has a density 1.84 g/cc at 35°C and contains 98% by weight is
(A) 1.84 M
(B) 18.4 M
(C) 20.6 M
(D) 24.5 M
110. If the elevation in boiling point of a solution of non-volatile, non-electrolytic and non-associating solute in a solvent (Kb = x K kg mol−1) is yK, then the depression in freezing point of solution of same concentration would be (Kf of the solvent = z K kg mol−1)
(A) 2xz/y
(B) yz/x
(C) xz/y
(D) yz/2x
111. The equivalent conductance of an aqueous solution of 1.0283 × 10−3 g equivalent acetic acid per litre is 48.5 Ω−1 cm2 equiv−1 at 25°C and at infinite dilution value is 390.7Ω−1 cm2 equive−1. Calculate the degree of ionization and ionization constant of acetic acid.
(A) 0.1232, 1.78 × 10−5
(B) 0.223, 10.2 × 10−5
(C) 0.229, 1.78 × 10−5
(D) 0.531, 2.85 × 10−5
112. At 25°C, the molar conductance of 0.007 M hydrofluoric acid is 150 mho cm2 mol−1 and its Λ°m = 500 mho cm2 mol−1. The value of the dissociation constant of the acid at the given concentration at 25°C is
(A) 7 × 10−5
(B) 7 × 10−5
(C) 9 × 10−3
(D) 9 × 10−4
113. In a galvanic cell, the salt bridge
(A) does not participate chemically in the cell reaction
(B) Stops the diffusion of ions from one electrode to another
(C) is necessary for the occurrence of the cell reaction
(D) ensures mixing of the two electrolytic solutions
114. 1 M solution each of Cu (NO3)2, AgNO3, Hg2 (NO3)2 and Mg(NO3)2 is electrolysed using Pt-electrodes. The values of standard electrode potentials in volts are Ag+/Ag = +0.80 V, Cu2+/Cu = +0.34V, Hg22+/Hg = +0.79 V, Mg2+/Mg = −37 V. The sequence of deposition of metals on the cathode will be
(A) Mg > Ag > Cu
(B) Mg > Cu > Ag
(C) Ag > Hg > Cu
(D) Cu > Hg > Ag
115. In the hydrolysis of an organic chloride in the presence of large excess of water, RCI + H2O → ROH + HCI
(A) Molecularity and order of reaction both are 2
(B) Molecularity is 2 but order of reaction is 1
(C) Molecularity is 1 but or reaction is 2
(D)Molecularity is 1 and order of reaction is also 1
116. For the non-stoichiometric reaction, 2A + B → C + D the following kinetic data were obtained in three separate experiments, all at 298 K.

The rate law for the formation of C is
(A) ![]()
(B) ![]()
(C) ![]()
(D) ![]()
117. Under the same reaction conditions, initial concentration of 1.386 mol dm−3 of a substance becomes half in 40 seconds and 20 seconds through 1st order and zero order kinetics, respectively, ratio(k1/k0) of the rate constants for first order (k1) and zero order (k0) of the reaction is
(A) 0.5 mol dm−3
(B) 1.0 mol dm−3
(C) 1.5 mol dm−3
(D) 2.0 mol dm−3
118. Which one of the followings is an example of homogenous catalysis?
(A) Manufacture of ammonia by Haber’s process.
(B) Manufacture of sulphuric acid by contact process.
(C) Hydrogenation of oil
(D) Hydrolysis of sucrose in presence of dilute hydrochloric acid
119. The process of converting hydrated alumina into anhydrous alumina is called
(A) roasting
(B) smelting
(C) dressing
(D) calcination
120. Carbon reduction is NOT used to obtain Cr and Mn because
(A) Process is not thermodynamically feasible
(B) Process is not economically feasible
(C) Interstitial compounds are formed by Cr and Mn with carbon at high temperature
(D) Cr and Mr are high melting metals
121. A radioactive atom ![]() emits two α-particles and one β-particle successively. The number of neutrons in the nucleus of the product will be
emits two α-particles and one β-particle successively. The number of neutrons in the nucleus of the product will be
(A) x – 4 – y
(B) x – y – 5
(C) x – y – 3
(D) x – y – 6
122. Which among the following group 16 elements exists in more than two allotropic states?
(A) Polonium
(B) Tellurium
(C) Selenium
(D) Oxygen
123. Which of the following statements is NOT true for hydrolysis of XeF6?
(A) XeOF6 is formed.
(B) XeO2F2 is formed.
(C) It is a redox reaction.
(D) XeO3 is formed
124. What are constituents of ‘Mischmetal’?
(A) La, Fe
(B) La, Ce
(C) Fe, Ce
(D) Ce, Cu
125. Which of the ions is colourless inspite of the presence of unpaired electrons?
(A) La3+
(B) Eu3+
(C) Gd3+
(D) Lu3+
126. Among the following complexes, the one which shows zero crystal field stabilization energy (CFSE) is
(A) [Mn(H2O)6]3+
(B) [Fe(H2O)6]3+
(C) [Co(H2O)6]2+
(D) [Co(H2O)6]3+
127. The ligand N(CH2CH2NH2)3 is
(A) tridentate
(B) pentadentate
(C) tetradentate
(D) hexadentate
128. In the following reaction ![]() The product ‘P’ is
The product ‘P’ is
(A) RCHO
(B) R2CHOEt
(C) R3CH
(D) RCH(OEt)2
129. When alkyl halide is heated with dry Ag2O, it produces
(A) ester
(B) ether
(C) ketone
(D) alcohol
130. Iodoform can be prepared from all EXCEPT
(A) ethyl methyl ketone
(B) isopropyl alcohol
(C) 3-methyl – 2 – butanone
(D) isobutyl alcohol
131. A compound containing two-OH groups attached with one carbon atom is unstable but which one of the followings is stable?
(A) 
(B) 
(C) 
(D) None of these
132. When glycerol is heated with KHSO4 it gives
(A) CH2 = CH – CH3
(B) CH2 = CH – CH2OH
(C) CH2 = CH – CHO
(D) CH2 = C = CH2
133. What are X and Y in the following reaction sequence, ![]()
(A) C2H5Cl, CH3CHO
(B) CH3CHO, CH3COOH
(C) CH3CHO, CCl3CHO
(D) C2H5Cl, CCl3CHO
134. Two aromatic compounds having formula C7H8O which easily identifiable by FeCl3 solution test (violet coloration) are
(A) o – cresol and benzyl alcohol
(B) m – cresol and p – cresol
(C) o – cresol and p – cresol
(D) methyl phenyl ether and benzyl alcohol
135. Positive Tollen’s test is NOT observed for
(A) 
(B) 
(C) 
(D) 
136. An organic compound A upon reaction with NH3 gives B. On heating, B gives C. C in presence of KOH reacts with Br2 to give CH3CH2NH2. A is
(A) (CH3)2 CH – COOH
(B) CH3CH2COOH
(C) CH3COOH
(D) CH3CH2CH2COOH
137. The number of structural isomers possible from the molecular formula C3H9N is
(A) 4
(B) 5
(C) 2
(D) 3
138. In the Hoffmann bromide degradation reaction, the number of moles of NaOH and Br2 used per mole of amine produced are
(A) for moles of NaOH and two moles of Br2
(B) two moles of NaOH and two moles of Br2
(C) four moles of NaOH one mole of Br2
(D) one mole of NaOH and one mole of Br2
139. Dissolving 120g of urea (mol. wt. = 60) in 1000 g of water gave a solution of density 1.15 g/mL. The molarity of the solution is
(A) 1.78 M
(B) 2.00 M
(C) 2.05 M
(D) 2.22 M
140. A gaseous hydrocarbon gives upon combustion, 0.72g of water and 3.08 g of CO2. The empirical formula of the hydrocarbon is
(A) C2H4
(B) C3H4
(C) C6H5
(D) C7H8
141. In the ground state of Cu+, the number of shells occupied, subshells occupied, filled orbitals and unpaired electrons respectively are
(A) 4,8,15,0
(B) 3,6,15,1
(C) 3,6,14,0
(D) 4,7,14,2
142. The energy absorbed each molecule (A2) of a substance is 4.4 × 10−19 J and bond energy per molecule is 4.0 × 10−19 The kinetic energy of the molecule per atom will be
(A) 2.0 × 10−20 J
(B) 2.2 × 10−19 J
(C) 2.0 × 10−19 J
(D) 4.0 × 10−19 J
143. Which one of the following orders represents the correct sequence of the increasing basic nature of the given oxides?
(A) Al2O < MgO < Na2O > K2O
(B) MgO < K2O < Al2O3 < Na2O
(C) Na2O < K2O < MgO < Al2O3
(D) K2O < Na2O < Al2O3 < MgO
144. Among the followings, the number of elements showing only one non-zero oxidation state is O, Cl, F, N, P Sn, Tl, Na, Ti-
(A) 1
(B) 2
(C) 3
(D) 4
145. The root mean square speed of the molecules of diatomic gas is u. When the temperature is doubled, the molecules dissociate into two atoms. The new rms speed of the atom is
(A) √2U
(B) U
(C) 2U
(D) 4U
146. Standard enthalpy of Vaporization ∆vspH° for water at 100°C is 40.66 kJ mol−1. The internal energy of vaporization of water at 100°C (in kJ mol−1) is (assume waster vapour to behave like an ideal gas)
(A) +37.56
(B) −43.76
(C) +43.76
(D) +40.66
147. For a reaction, ∆H = +29 kJ mol−1, ∆S = −35 kJ mol−1. At what temperature the reaction will be spontaneous?
(A) 828.7°C
(B) 828.7K
(C) Spontaneous at all temperature
(D) Not possible
148. For the gas phase reaction,
C2H4 + H2 + C2H6; (∆H = −32.7 Kcal) carried out in a vessel, the equilibrium concentration of C2H4 can be increased by
(A) decreasing the pressure
(B) increasing the temperature
(C) removing some C2H6
(D) adding some H2
149. In which of the following equilibrium systems, is the rate of the backward reaction favoured by increase of pressure?
(A) PCl5 + PCl + Cl2
(B) 2SO2 + O2 + 2SO3
(C) N2 + 3H2 + 2NH3
(D) N2 + O2 + 2NO
150. At 25°C, the dissociation constants of CH3COOH and NH4OH in aqueous solution are almost the same, i.e., 10−5. If pH of some acetic acid solution is 3, the pH of NH4OH solution of same concentration at the same temperature would be
(A) 3.0
(B) 4.0
(C) 10.0
(D) 11.0
MATHEMATICS
151. 5th term from the end in the expansion of  is
is
(A) 25x2
(B) −70
(C) 70
(D) −25x2
152. If the co-efficients of x7 and x8 in  are equal, then value of n is
are equal, then value of n is
(A) 56
(B) 55
(C) 47
(D) 19
153. If a is a real number and if the middle term of 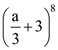 is 1120, then value of a is
is 1120, then value of a is
(A) ±2
(B) ±1
(C) ±√3
(D) ±√2
154. Suppose a1, a2……are in A.P. if a8 : a5 = 3 : 2 then a17 : a23 is
(A) 1 : 2
(B) 3 : 4
(C) 4 : 11
(D) 8 : 11
155. Suppose a, b, c > 0 and p ∈ If(a2 + b2)p2 – 2 (ab + bc)p + (b2 + c2) = 0 then a, b, c are in
(A) A.P
(B) G.P
(C) H.P
(D) A.G.P
156. Three positive numbers form an increasing G.P. If the middle term in this G.P. is tripled, the new numbers are in A.P. Then the common ratio of G.P. is
(A) 3 + 2√2
(B) 2√2 – √3
(C) 2 + 2√3
(D) 2√2 + √3
157. ![]()
(A) 1/6
(B) −1/12
(C) 2/3
(D) 1/3
158. If ![]() then the value of a is
then the value of a is
(A) −7
(B) −1
(C) 7
(D) None of these
159. The value of ![]()
![]() Where α and β are the roots of ax2 + bx + c = 0 is
Where α and β are the roots of ax2 + bx + c = 0 is
(A) (a – b)2
(B) ![]()
(C) ![]()
(D) None of these
160. If x = k(t – sin t), y = k(1 – cos t) (k ≠ 0) the ![]() is equal to
is equal to
(A) −1/k
(B) 1/2k
(C) 1/k2
(D) 2k
161. If ![]() and
and ![]() then
then ![]() is equal to
is equal to
(A) 1/x3
(B) 2/x3
(C) 2√2/x3
(D) 2√2/x4
162. If function f(x) is differentiable at x = a then  is
is
(A) −a2fʹ(a)
(B) af(a) – a2fʹ(a)
(C) 2af(a) – a2fʹ(a)
(D) 2af(a) + a2fʹ(a)
163. If y = cosec (cot−1 x) then dy/dx at x = 1 is equal to
(A) 1/√2
(B) 1
(C) −√2
(D) −1/√2
164. If ![]() then its antiderivative F(x) is given
then its antiderivative F(x) is given
(A) ![]()
(B) ![]()
(C) ![]()
(D) ![]()
165. If ![]() then f(x) is equal to
then f(x) is equal to
(A) ![]()
(B) ![]()
(C) ![]()
(D) ![]()
166. Let ![]() and F(x) is this antiderivative, if F(π/4) = 6 then F(x) is equal to
and F(x) is this antiderivative, if F(π/4) = 6 then F(x) is equal to
(A) ![]()
(B) ![]()
(C) 
(D) None of these
167. The integral  is equal to
is equal to
(A) 
(B) 
(C) ![]()
(D) None of these
168. The value of ![]() is
is
(A) 0
(B) 1
(C) π/4
(D) π/2
169. The solution of the equation ![]() is given by
is given by
(A) ![]()
(B) ![]()
(C) ![]()
(D) ![]()
170. A particular solution of ![]() is given by
is given by
(A) 5e4x + 4e−5y = 9
(B) 5e4x + 4e5y = 9
(C) 4e4x + 5e−4x = 20
(D) 4e4x + 5e−4e = 9
171. The order and degree of the different equation  are (respectively)
are (respectively)
(A) 2, 1
(B) 2, 3
(C) 2, 2
(D) 2, 6
172. If the lines 3x – y + 1 = 0 and x – 2y + 3 = 0 are equally inclined to the line y = mx, then the value of m is given by
(A) 2m2 – 7 – 7 = 0
(B) 7m2 – 7m – 2 = 0
(C) 7m2 – 7m – 2 = 0
(D) 2m2 – 7m – 2 = 0
173. A ray of light passing through the point (3, 7) reflects of the X-axis at a point A and the reflected ray passes through the point (2, 5), the coordinates of A are
(A) (29/12, 0)
(B) (1/2, 0)
(C) (−1/2, 0)
(D) (−29/12, 0)
174. If p is the length of the perpendicular from the origin on the line ![]() and a2, p2, b2 are in A.P., then a4 – 2p2a2 + 2p4 = ?
and a2, p2, b2 are in A.P., then a4 – 2p2a2 + 2p4 = ?
(A) −1
(B) 0
(C) 1
(D) None of these
175. The locus of the point P(h, K), when the area of the triangle formed by the lines y = x, x + y = 2 and the line through P(h, k) and parallel to the X-axis is 4h2 is
(A) x + 2y – 1 = 0
(B) 2x + y – 1 = 0
(C) 2x – y – 1 = 0
(D) x – 2y + 1 = 0
176. If a circle passes through the point (3, 4) and cuts the circle x2 + y2 = a2 orthogonally, the equation of the locus of its centre is
(A) 3x + 4y – a2 = 0
(B) 6x + 8y = a2 + 25
(C) 6x + 8y + a2 + 25 = 0
(D) 3x + 4y = a2 + 25
177. If α, β are accentric angles of the extremities of a focal chord of the ellipse ![]() then tan (α/2) tan(β/2) = ?
then tan (α/2) tan(β/2) = ?
(A) ![]()
(B) ![]()
(C) ![]()
(D) ![]()
178. The ratio in which the plane 2x – 1 = 0 divides the line joining (−2, 4, 7) and (3, −5, 8) is
(A) 2 : 3
(B) 4 : 5
(C) 7 : 8
(D) 1 : 1
179. Cosine of the angle between the lines whose vector equation are r = 3i + 2j – 4k + λ(i + 2j + 2k) and r = 5i – 2k + μ(3i + 2j + 6k) : λ, μ being parameters, is
(A) −1/3√29
(B) 3/7√29
(C) 23/29
(D) 19/21
180. If a, b, c are non-coplanar unit vectors such that a ![]() then the angle between a and b is
then the angle between a and b is
(A) 3π/4
(B) π/4
(C) π/2
(D) π
181. The variance of first 20-natural number is
(A) 133/4
(B) 379/12
(C) 133/2
(D) 399/4
182. For two data sets, each of size 5, the variance are given to be 4 and 5, the corresponding means are given to be 2 and 4 respectively. The variance of the combined data set is
(A) 6
(B) 13/2
(C) 5/2
(D) 11/2
183. If 15 boys of different ages are distributed into 3 groups of 4, 5 and 6 boys randomly then the probability that three youngest boys are in different groups is
(A) 24/91
(B) 71/91
(C) 67/91
(D) 20/91
184. Let ![]()
![]() is equal to
is equal to
(A) 2 + √3
(B) 2 – √3
(C) √3 + 1
(D) √3 – 1
185. If ![]() then
then ![]() is equal to
is equal to
(A) ![]()
(B) ![]()
(C) 1/k2
(D) a/k
186. The value of ![]() is equal to
is equal to
(A) 3/4
(B) 5/4
(C) 5/2
(D) 4/5
187. ![]() if tan θ is equal to
if tan θ is equal to
(A) −2
(B) −1
(C) 2/3
(D) 2
188. Two flagstaffs stand on a horizontal plane. A and B are two points on the lie joining their feet and between them The angles of elevation of the tops of the flag staffs as seen from A are 30° and 60° and as seen from B are 60° and 45°. If AB is 30 m, the distance between the flagstaffs in meters is
(A) 30 + 15√3
(B) 45 + 15√3
(C) 60 – 15√3
(D) 60 + 15√3
189. The domain of ![]() is
is
(A) [−3/2, 5/2]
(B) [−1, 1]
(C) [0, 2]
(D) [−1/2, 3/2]
190. Which of the following functions is an odd function?
(A) y = x4 – 2x2
(B) y = x – x2
(C) y = cos x
(D) ![]()
191. If ω(≠1) is a cube root of unity and (1 + ω2)11 = a + bω + cω2, then (a, b, c) equals
(A) (1, 1, 0)
(B) (0, 1, 1)
(C) (1, 0, 1)
(D) (1, 1, 1)
192. If  then
then
(A) Re(z) = 0
(B) 1m(z) = 0
(C) Re(z) > 0, 1m(z) < 0
(D) Re(z) < 0, 1m (z) > 0
193. If ![]() then |z| CANNOT exceed
then |z| CANNOT exceed
(A) 3
(B) 8
(C) 16
(D) 18
194. Value of ![]()
(A) 3
(B) 2
(C) 1
(D) None of these
195. Let x, y, z be positive and x, y, z ≠ 1, Let  then numerical value of ∆ is
then numerical value of ∆ is
(A) −1
(B) 0
(C) 1
(D) None of these
196. If ![]() the matrix A equals
the matrix A equals
(A) 
(B) 
(C) 
(D) 
197. If  then A2 – 5A – 1 equals
then A2 – 5A – 1 equals
(A) 0
(B) I
(C) 2 I
(D) None of these
198. The value of  is
is
(A) 1
(B) 36C17
(C) 2/19
(D) 36C18
199. If nCr : nCr+1 :nCr+2 = 1 : 2 : 3, then r is equal to
(A) 5
(B) 4
(C) 3
(D) 0
200. The value of  is
is
(A) 56C3
(B) 56C4
(C) 55C4
(D) 56C3
BIOLOGY
151. DNA synthesis can be specifically measured by estimating the incorporation by radia labelled
(A) Uracil
(B) Adenine
(C) Thymidine
(D) Deoxyribose sugar
152. The enzyme reverse transcriptase is
(A) RNA dependent RNA polymerase
(B) RNA dependent DNA polymerase
(C) DNA dependent DNA polymerase
(D) DNA dependent RNA polymerase
153. Which of the following hydrolyeses internal phosphiodiester bonds in a polynucleotide chain?
(A) Lipase
(B) Ligase
(C) Exonuclease
(D) Endonuclease
154. Find the sequence of binding of the following amine acyl t-RNA complexes during translation to a m-RNA transcribed by a DNA segment having the base sequence ‘3TACATGGGTCCG5’. Choose the answer showing the correct order of numerals.
(i) AUG (ii) UAC (iii) CCG (iv) GGU
(A) (ii), (i), (iv), (iii)
(B) (i), (ii), (iv), (iii)
(C) (ii), (i), (iii), (iv)
(D) (i), (ii), (iii), (iv)
155. How many types of gametes will be produced in the following allelic sequences Aa BB CC Dd Ee?
(A) 64
(B) 32
(C) 8
(D) 16
156. What will happen if the secretion of parietal cells of the gastric glands is blocked with an inhibitor?
(A) Gastric juice will be deficient in pepsinogen.
(B) Gastric juice will be deficient in chymsin.
(C) In the absence of HCI secretion inactive pepsinogen is not converted into pepsin.
(D) Enterokinase will not be released.
157. What is TRUE about haemoglobin?
(A) It is a dipeptide and present in red blood corpuscles in blood worm.
(B) It is present in the dissolved state in blood plasma in earth worm.
(C) It is a dipeptide in mammals and localized in RBC.
(D) It is present in dissolved state in blood plasma in Scorpion
158. In which of the following organs will the rate of blood flow change the least during exercise?
(A) Heart
(B) Intestine
(C) Brain
(D) Skin
159. Find the INCORRECT statement regarding mechanism of urine formation in man.
(A) Tubular secretion takes place in the PCT.
(B) Aldosterone induces greater reabsorption of sodium
(C) The counter current systems contribute in diluting the urine.
(D) The ultra filtration is opposed by the colloidal osmotic pressure of plasma.
160. Identify the TRUE statement in the following.
(A) Each nucelosome consists of a core of five types of nine histone molecules.
(B) Oxidation of fatty acids and synthesis of phosopholipids occur in peroxisomes.
(C) Telocentric chromosome contains two unequal arms.
(D) Smaller subunit of ribosome contains the enzyme peptidyl transferase.
161. Cellulose, the most important constituent of plant cell well is made of
(A) unbranched chain of glucose molecules linked b y β-1, 4-glycoside bond
(B) branched chain of glucose molecules linked by α-1, 6-glycosidic bond at the site of branching
(C) unbranched chain of glucose molecules lined by α-1, 4-glycosidic bond
(D) branched chain of glucose molecules linked by β-1, 4-glycosidic bond in straight chain & α-1, 6-glycosidic bond at the site of branching
162. Colchicine prevents the mitosis of cells at which of the following stages?
(A) Anaphase
(B) Metaphase
(C) Prophase
(D) Interphase
163. Which one of the following is NOT associated with ascent of sap in tall trees?
(A) Cohesion and adhesion of water molecules
(B) Continuity of water column
(C) Pressure in tracheary elements
(D) Transpiraationpull
164. Stomatal opening is affected by
(A) nitrogen, carbon-dioxide concentration and light
(B) carbon-dioxide concentration, temperature and light
(C) nitrogen concentration, temperature and light
(D) nitrogen, carbon-dioxide concentration and temperature
165. C4 plants have higher net photosynthetic rate because
(A) they have no photorespiration
(B) they can photosynthesize in low light intensity
(C) they have PEP as CO2 acceptor
(D) they have Kranz type of antomy
166. Which statement is WRONG for Kreb’s Cycles?
(A) There is one point in the cycle where FAD+ is reduced to FADH2.
(B) During conversion of succinyl CO-A to succinic acid a molecule of GTP is synthesized.
(C) The cycle starts with condensation of acertyl group (acetyl CoA) with pyruvic acid to yield citric acid.
(D) There are three points in the cycle where NAD+ is reduced to NADH+H+.
167. Match each disease with its correct type of vaccine.
Disease Vaccine
(a) Tuberculosis (i) Harmless virus
(b) Whooping cought (ii) Killed bacteria
(c) Diphteria (iii) G2-phase
(d) Polio (iv) Harmless bacteria
(A) (a)-(ii), (b)-(i), (c)-(iii), (d)-(iv)
(B) (a)-(iii), (b)-(ii), (c)-(iv), (d)-(i)
(C) (a)-(iv), (b)-(iii), (c)-(ii), (d)-(i)
(D) (a)-(i), (b)-(ii), (c)-(iv), (d)-(iii)
168. Myelin sheath is produced by
(A) astorcytes and schawann cells
(B) Oligodendrocytes and osteoclasts
(C) osteoclasts and astrocytes
(D) schewann cells and oligodendrocyts
169. Column-I is the part of human brain & Column –II is its function. Match the two columns.
Column-I Column-II
(a) Cerebrum (p) Controls the pituitary
(b) Cerebelum (q) Controls vision and hearing
(c) Hypothalamus (r) Controls the rate of hearing
(d) Midbrain (s) Site of intelligence
(t) Maintains body posture
(A) (a)-(s), (b)-(t), (c)-(p), (d)-(q)
(B) (a)-(t), (b)-(s), (c)-(q), (d)-(p)
(C) (a)-(s), (b)-(t), (c)-(q), (d)-(p)
(D) (a)-(t), (b)-(s), (c)-(p), (d)-(r)
170. Select the correct matched pair.
(A) Pineal gland-does not influence menstrual cycle
(B) Corpus luteum-secrets oxytocin
(C) Interstitial cells-erythropoietin
(D) Cholecystokinin-stimulates contraction of gall bladder
171. How many ova and sperms will be produced from 100 secondary oocytes and 100 secondary spermatocytes during gametogenesis in man?
(A) 50 ova, 100 sperms
(B) 100 ova, 100 sperms
(C) 200 ova, 200 sperms
(D) 100 ova, 200 sperms
172. ‘Shaeli’ a new oral contraceptive for the female developed by Indian scientist a
(A) steroidal preparation
(B) hormonal preparation
(C) non-sterodial preparation
(D) toxic preparation to kill sperms
173. Which of these is correct hierarchial order?
(A) Division-Order-Family-Class
(B) Class-Family-Order-Division
(C) Family-Order-Class-Division
(D) Order-Class-Family-Division
174. Teichoic acid is present in the cell wall of
(A) Gram +ve bacteria
(B) Gram – ve bacteria
(C) Cyanobacteria
(D) Mycoplasma
175. A person suffering from a disease caused by plasmodium experiences recurring chill and fever at the time when
(A) the sporozoites released from RBCs are being rapidly killed and broken down inside spleen.
(B) the trophozoites reach maximum growth and give out certain toxins
(C) the parasite after its rapid multiplication inside RBCs ruptures them, releasing the stage to enter fresh RBCs
(D) the microgametocytes and megagametocytes are being destroyed by the WBCs
176. Edible part of Mushroom is
(A) Mycelium
(B) Basidiocarp
(C) Basidiospores
(D) Fungal hyphae
177. Which one of the following is NOT a characteristic features of Bryophytes?
(A) Dominant gametophytic generation
(B) Flamentous rhizoids
(C) Amphibious habitat
(D) Vascular tissues
178. Coal is formed by
(A) Pteridophytes
(B) Bryophytes
(C) Fungi
(D) Algae
179. Find out the WRONGLY matched pair.
(A) Tuber – Potato
(B) Rhizome – Ginger
(C) Bulbil – Agave
(D) Leaf buds – Banana
180. Starch is mainly manufactured by
(A) Palisade parenchyma
(B) Spongy parenchyma
(C) Guard cells
(D) Vascular bundle
181. In most simple type of canal system of porifera water flows through which one of the following ways?
(A) Spongocuel → Ostia → Osculum → Exterior
(B) Ostia → Spongocoel → Osculum → Exterior
(C) Osculum → Spongocoel → Ostia → Exterior
(D) Osculum → Ostia → Spongocoel → Exterior
182. Which of the following is NOT correctly matched?
(A) Ascaris – Flame cell
(B) Prawn – Haemocoel
(C) Sycon – Canal system
(D) Star fish – Radial symmetry
183. The correct sequence of arrangement of segments in the leg of cockroach is
(A) Coxa, Femur, Trochanter, Tibia, Tarsus
(B) Coxa, Trochanter, Femusr, Tibia, Tarsus
(C) Trochanter, Coxa, Tibia, Femur, Tarsus
(D) Trochanter, Coxa, Femur, Tibia, Tarsus
184. All mammals without any exception are characterized by
(A) viviparity and biconcave RBC
(B) heterodont teeth & 12 pairs of cranial nerves
(C) a muscular diaphragm and milk producing glands
(D) extra abdominal testes and a four chambered heart
185. Which is the principal cation in the plasma of blood?
(A) Calcium
(B) Sodium
(C) Potassium
(D) Magnesium
186. Microfilaments are composed mainly of a protein called
(A) Actin
(B) Myosin
(C) Tubulin
(D) Chitin
187. Match the following & choose the correct combination from the option given below.
Column-I Column-II
(a) Sulphur (1) Chlorophy II
(b) Zinc (2) Nitrogen
(c) Magnesium (3) Methionine
(d) Molybdenum (4) Auxin
(A) (a) – (1), (b) – (2), (c) – (3), (d) – (4)
(B) (a) – (3), (b) – (4), (c) – (1), (d) – (2)
(C) (a) – (3), (b) – (1), (c) – (2), (d) – (4)
(D) (a) – (2), (b) – (4), (c) – (1), (d) – (3)
188. Miller synthesized simple amino acids from one of the following mixtures in an experiment.
(A) H2, O2, N2 (1 : 2 : 1) and water vapour
(B) H2, O2, N2 (2 : 1 : 2) and water vapour
(C) CH4, NH3, H2(2 : 1 : 2) and water vapour
(D) CH4, NH3, H2 (1 : 2 : 1) and water vapour
189. ‘Muscular dystrophy’ is caused by eating Lathyrus sativus. This is due to presence of
(A) Allyl sulphide
(B) Trans cinnamic acid
(C) β-oxalyl amino alanine
(D) Cucuribitacin
190. Photochemical smog is caused by light mediated reaction between
(A) NO2 and O3
(B) NO2 and unsaturated hydrocarbon
(C) SO3 and O3
(D) SO3 and unsaturated hydrocarbon
191. As it travels along the food-chain the concentration of DDT
(A) increases
(B) decreases
(C) stays constant
(D) Fluctuates randomly
192. Match the following:
I-Helophytes, II-Psammophytes, III-Oxylophytes, IV-Chasmophytes
A-Saline soil, B-Sandy soil, C-Rock crevices, D-Acidic soil
(A) I-A, II-B, III-D, IV-C
(B) I-A, II-C, III-D, IV-C
(C) I-A, II-B, III-C, IV-D
(D) I-D, II-C, III-B, IV-A
193. Tobacco plants resistant to a nematode have seen developed by the introducing of DNA that produced (in host cells)
(A) both sense and antisense RNA
(B) a particular hormone
(C) an antifeedant
(D) a toxin protein
194. Consider the following four statements (1-4) and select the option which includes all the correct ones only.
(1) Single cell spirulina can produce large quantities of food rich in protein, minerals, vitamins etc.
(2) Body weight-wise the micro-organism Methylophilus methylotrophys may be able to produce several times more protein than the other organism.
(3) A rice variety has been developed which is very rich in calcium.
(4) Common button mushrooms are a very rich source of vitamin C.
(A) Statements (1) and (2)
(B) Statements (3) and (4)
(C) Statements (1), (3) and (4)
(D) Statements (2), (3) and (4)
195. A recombinant DNA molecule can be produced in the absence of the following.
(A) Restriction endonuclease
(B) DNA ligase
(C) DNA fragments
(D) E. Coli
196. A technology which has found immense use in solving cases of disputed parentage, is
(A) Polymerase chain reaction
(B) DNA finger printing
(C) Monoclonal antibody production
(D) Recombinant DNA technology
197. Antibiotics inhibit the growth of or destroy
(A) bacteria & fungi
(B) bacteria & viruses
(C) bacteria, algae & viruses
(D) bacteria, fungi & viruses
198. Uncontrolled plant introduction is responsible for introduction of which weed in India?
(A) Agremone mexicana
(B) Lantana camara
(C) Eichhornia crassipes
(D) All of these
199. Porous wood is generally of
(A) Angiosperms
(B) Gymnosperms
(C) Both (A) and (B)
(D) None of these
200. Non-alcoholic beverage contains stimulating property due to the presence of
(A) Caffeine
(B) Atropine
(C) Nimbin
(D) Thymol
Latest Govt Job & Exam Updates: