| Go to all OUAT Previous Year Question Papers | 2022, 2021, 2020, 2019, 2018, 2017, 2016, 2015, 2014, 2013, 2012,2011, 2010, 2009, 2008, 2007 |
OUAT Previous Question Paper-2020
PHYSICS
(Questions 1-66)
1. A particle is moving on a straight line path with constant acceleration directed along the directions of instantaneous velocity. Which of the following statements are FALSE about the motion of particle?
(A) Particle may reverse the direction of motion.
(B) Distance covered is not equal magnitude of displacement.
(C) The magnitude of average velocity is less than average speed.
(D) All of these
2. In photoelectric effect, work function of material is 3.5 eV. By applying −2 V potential, photoelectric current becomes zero, so
(A) Energy of incident photon is 4.74 eV.
(B) Energy of incident photon is 2.3 eV.
(C) If photon having higher frequency is used, photoelectric current is produced.
(D) When energy of photon is 2.3 eV, photoelectric current becomes maximum.
3. A conducting ring is placed around the core of an electromagnet as shown in figure. When ket K is pressed, the ring

(A) remains stationary
(B) is attracted towards the electromagnet
(C) jumps out the core
(D) None of these
4. The height of the building is 50 ft. The same in millimeter is
(A) 560 mm
(B) 285 mm
(C) 1786.8 mm
(D) 15240 mm
5. In the following diagrams, all the charges have equal magnitude. Electric field is zero at the centre of

6. If 20 V battery is connected to primary coil of a transformer, then output voltage is
(A) zero
(B) 20V
(C) 10 V
(D) None of these
7. Mean kinetic energy per gm. Molecule of a gas is given by
(A) ![]()
(B) kT
(C) ![]()
(D) ![]()
8. A hollow sphere is filled with water. There is a hole at the bottom of this sphere. This sphere is suspended with a string from a rigid support and given an oscillation. During oscillation, the hole is opened up and the periodic time of this oscillating system is measured. The periodic time of the system.
(A) will remain constant.
(B) will increase upto a certain time.
(C) increases initially and then decreases to attain its initial periodic time.
(D) initially decreases and then will attain the initial periodic time value.
9. The amount of heat generated in 500 Ω resistance, when the key is thrown over from constant 1 to 2, as shown in figure is
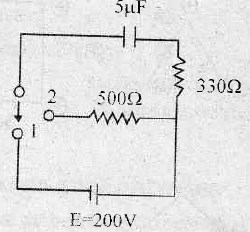
(A) 40 × 10−3 J
(B) 50 × 10−3 J
(C) 60 × 10−3 J
(D) 30 × 10−3 J
10. In the given figure, the convex lens is cut into two pieces and displace along the axes for small distance. The shape of fringe formed on the screen is

(A) elliptical
(B) hyperbolic
(C) circular
(D) None of these
11. Two point charges A and B of same charge having magnitude of momenta p1 and p2 respectively and having same charge are moving in a plane containing uniform magnetic field perpendicular to the plane. Then (Trajectories as shown in figure).

(A) p1 = p2
(B) p1 > p2
(C) p1 < p2
(D) None of these
12. carrier wave is modulated by n number of sine wave with modulation indices μ1, μ2 , μ3 ……. The total modulation index (μ) of the wave is
(A) μ1 + μ2 + μ3 +……
(B) ![]()
(C) 
(D) 
13. Which of the following quantities is NOT dimensionless?
(A) Reynold’s number
(B) Strain
(C) Angle
(D) Radius of gyration
14. Which one of the following diagrams correctly represents the energy levels in the p-type semi-conductor?
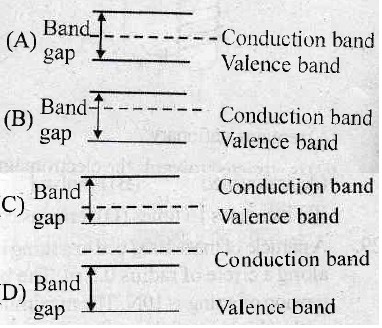
15. A wire of cross-section 4 mm2 is stretched by 0.1 mm by a certain weight. How far (length) will be wire of same material and length but of area 8 mm2 stretched under the action of same force.
(A) 0.05 mm
(B) 0.10 mm
(C) 0.15 mm
(D) 0.20 mm
16. An iron rod is subjected to cycles of magnetization at the rate of 50 Hz. Given the density of the rod is 8 × 103 kg/m3 and specific heat is 0.11 × 103 cal/Kg° The rise in temperature per minute, if the area inclosed by the B-H loop corresponds to energy of 10−2 J, is [Assume there is not radiation losses]
(A) 78°C
(B) 88°C
(C) 8.1°C
(D) None of these
17. Which one of the following is the correct graph between energy and wavelength for given photon?
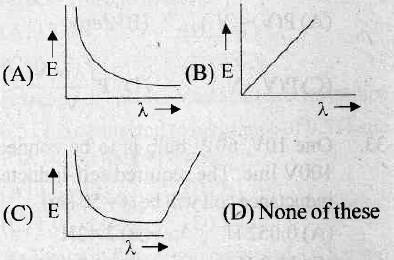
18. A coil of wire of radius R has 200 turns and a self-inductance of 108 mH. The self-inductance of a similar coil of 500 turns will be
(A) 375 mH
(B) 527 mH
(C) 675 mH
(D) None of these
19. de-Broglie wavelength of atom at T k absolute temperature will be
(A) h/mkT
(B) h/√3mkT
(C) √2mkT/h
(D) √2mkT
20. A glass ball is dropped from height 10m. If there is 20% loss of energy due to impact, then after one impact, the ball will go upto
(A) 2 m
(B) 4 m
(C) 6 m
(D) 8 m
21. The breaking stress of wire of length 1 and radius is 5 kgwtm−2. The length and radius of wire are doubled, the breaking stress in kgwtm−2 is
(A) 5
(B) 10
(C) 20
(D) 80
22. Mark correct option:
(A) The root mean square speeds of the molecules of different ideal gases, maintained at the same temperature are the same.
(B) Electrons in a conductor have no motion in the absence of a potential difference across it.
(C) One mole of a monoatomic ideal gas is mixed with one mole of a diatomic ideal gas. The molar specific heat of the mixture at constant volume is 2R.
(D) The pressure exerted by an enclosed ideal gas depends on the shape of t he container.
23. The current is resistance R at resonance is

(A) zero
(B) minimum but finite
(C) maximum but finite
(D) infinite
24. In Young’s double slits experiment, the length of band is 1 mm. The fringe width is 0.021 mm. The number of fringes is

(A) 45
(B) 46
(C) 47
(D) 48
25. A block is released from top of a smooth inclined plane. It reaches the bottom of the plane is 6 sec. The time taken by the body to cover the first half of the inclined plane is
(A) 3 sec
(B) 4 sec
(C) 3√2 sec
(D) 5 sec
26. Hailstone at 0°C falls from a height of 1 km on an insulating surface converting whole of its kinetic energy into heat. What part of it will melt ? (g = 10 m/s2)
(A) 1/33
(B) 1/8
(C) 1/33 × 10−4
(D) All of it will melt
27. Deuterium atoms in the ground state are radiated by photons of energy 12.8 eV. What will be the energy of induced radiation of longest wavelength? Ionisation energy of deuterium is 14.4 eV.
(A) 12.8 eV
(B) 10.8 eV
(C) 1.6 eV
(D) 2.0 eV
28. If both the length of an antenna and the wave length of the signal to be transmitted are doubled, the power radiated by the antenna
(A) is doubled
(B) is halved
(C) increases 16 times
(D) remains constant
29. A particle of mass 100g tied to a string is rotated along a circle of radius 0.5 m. The breaking rension of string is 10 N. The maximum speed with which particle can be rotated without breaking the string is
(A) 10 m/s
(B) 9.8 m/s
(C) 7.7 m/s
(D) 7.07 m/s
30. A particular nucleus in a large population of identical radioactive nuclei survives 10 half lives of that isotope. The probability that this surviving nucleus will survive the next half-life is
(A) 1/10
(B) 2/5
(C) 1/2
(D) 1/210
30. A 50 mH coil carries a current of 2 amp, the energy stored in joule is
(A) 1
(B) 0.05
(C) 0.1
(D) 0.5
32. A gas expands under constant pressure P from volume V1 to V2, the work done by the gas is
(A) P(V1 – V2)
(B) Zero
(C) P(V1 + V2)
(D) 
33. One 10V, 60W bulb is to be connected to 100V line. The required self-inductance of inductance coil will be (f = 50 Hz)
(A) 0.052 H
(B) 2.42 H
(C) 16.2 H
(D) 16.2 mH
34. As shown in figure, a body having mass m is attached with two springs having spring constants k1 and k2. The frequency of oscillation is f. Now, if the springs constants of both the springs are increased 4 times, then the frequency of oscillation will be equal to

(A) 2f
(B) f/2
(C) f/4
(D) 4f
35. An isolated solid metallic sphere is given +Q charge. The charge will be distributed on the sphere
(A) uniformly but only on surface.
(B) only on surface but non-uniformly
(C) uniformly inside the volume.
(D) non-uniformly inside the volume
36. At constant volume temperature is increased then
(A) collision walls will be less
(B) collision frequency will increase
(C) collision will be in straight line
(D) collision will not change
37. Minimum excitation potential of Bohr’s first obrbit in hydrogen atom is
(A) 13.6 V
(B) 3.4 V
(C) 10.2 V
(D) 3.6 V
38. A battery of emf 1.2 V and internal resistance 0.5Ω is connected to resistance of 0.5 Ω the P.D. across the resistor is
(A) 1.2 volt
(B) 1.1 volt
(C) 1.05 volt
(D) 1 volt
39. As shown in figure, two masses of 3.0 k.g and 1.0 kg are attached at the two ends of a spring having force constant 300 Nm−1. The natural frequency of oscillation for the system will be ______ hz. (Ignore friction)

(A) 1/4
(B) 1/3
(C) 4
(D) 3
40. A gas is taken in a sealed container at 300 K. It is heated at constant volume to a temperature 600 K. The mean K. E. of its molecules is
(A) halved
(B) doubled
(C) tripled
(upled.0
41. A wire of diameter 1 mm breaks under a tension of 100N. Another wire of same materials as that of the first one, but of diameter 2 mm breaks under a tension of
(A) 500 N
(B) 1000 N
(C) 10,000 N
(D) 4000 N
42. If the total magnetic field due to the earth is 28 Am−1 then the total magnetic induction due to the earth is
(A) 3.52 × 10−7 T
(B) 3.52 × 10−5 T
(C) 3.52 × 10−2 T
(D) 3.52 × 10−4 T
43. Kinetic energy of emitted ray is dependent on
(A) voltage only
(B) work function only
(C) Both (A) and (B)
(D) It does not depend upon physical quantity
44. How many photons are emitted by a laser source of 5 × 10−3 W operating at 632.2 nm in 2 second ? (ft = 6.63 × 10−34 Js)
(A) 3.2 × 10−16
(B) 1.6 × 10−16
(C) 4. × 10−16
(D) None of these
45. In a tangent galvanometer, a current of 01 A produces a deflection of 30°. The current required to produce a deflection of 60°, is
(A) 0.2 A
(B) 0.3 A
(C) 0.4 A
(D) 0.5 A
46. When a rubber cord is stretched, the change in volume with respect to change in its linear dimension is negligible, the Poisson’s ratio for rubber is
(A) 1
(B) 0.25
(C) 0.5
(D) 0.75
47. Choose the correct order of the root mean square velocity (υrms), the average velocity (υav) and the most probable velocity (υmp).
(A) υmp > υav > υrms
(B) υrms > υav > υmp
(C) υav > υmp > υrms
(D) υmp > υrms > υav
48. A block of mass 100g slides on a rough horizontal surface. If the speed of the block decreases from 10 m/s to 5 m/s, the thermal energy developed in the process is
(A) 3.75 J
(B) 37.5 J
(C) 0.375 J
(D) 0.75 J
49. In order to obtain time constant of 10 second in an R-C circuit containing a resistance of 103 Ω, the capacity of the condenser should be
(A) 10 μF
(B) 100 μF
(C) 1000 μF
(D) 10000 μF
50. A conducting circular loop of radius r carries constant current i. It is placed in a uniform magnetic field B0 such that B0 is magnitude of magnetic field to a plane of the loop, the magnetic force acting on the loop is
(A) irB0
(B) 2πirB0
(C) πirB0
(D) Zero
51. In the figure three identical springs are shown. From spring A, a mass of 4 kg is hung and spring shows elongation of 1 cm. But when a weight of 6 kg is hung on B, the Hook’s descends
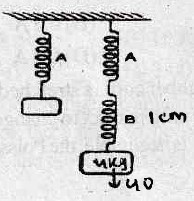
(A) 1 cm
(B) 2 cm
(C) 3 cm
(D) 4 cm
52. A planet is moving in an elliptical orbit. If T, V, E and L are respectively the kinetic energy, potential energy, total energy and the magnitude of the angular momentum of the planet then the TRUE statement out of the following is
(A) T is conserved
(B) V is always positive
(C) E is always negative
(D) L is conserved but the direction vectr L continuously changes
53. In the given graph, adiabatic and isothermal curves are shown:

(A) the curve A is isothermal
(B) the curve B is isothermal
(C) the curve A is adiabatic
(D) Both (B) and (C) are correct
54. Light of wavelength λ is incident on a slit of width d and distance between screen and slit is D. Then width of maxima and width of slit will be equal, if D is
(A) d2/ λ
(B) 2d/ λ
(C) 2d2/ λ
(D) d2/ 2λ
55. The gravitational potential difference between the surface of a planet and a point 20 m above it is 16 J/kg. Then the work done in moving a 2kg mass by 8m on
(A) 11.1 J
(B) 5.55 J
(C) 16 J
(D) 27.7 J
56. A particle of mass m is attached to one end of a string of length l while the other end is fixed to point h above the horizontal table, the particle is made to revolve in a circle on the table, so as to make P revolutions per second. The maximum value of P if the particle is to be in contact with the table will be
(A) ![]()
(B) 
(C) 
(D) 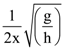
57. The alpha and beta particles cause ionization because of
(A) photoelectric emission
(B) Compton collision
(C) pair production
(D) the electrostatic force
58. force of 0.5 is applied on upper block as shown in figure. The work done by lower block on upper block for displacement 3m of the upper block is
(Take g = 10 m/s2)

(A) 1 joule
(B) −1 joule
(C) 2 joule
(D) −2 joule
59. Figure shown a block of mass m kept on inclined plane with inclination θ. The tension in the string is

(A) 8 N
(B) 10 N
(C) 0.8 N
(D) Zero
60. A train starts from station with an acceleration 1 m/s2. A boy who is 48 m behind the train with a constant velocity 10 m/s, the minimum time after which the boy will catch the train is
(A) 4.8 sec
(B) 8 sec
(C) 10 sec
(D) 12 sec
61. In an elastic string whose natural length is equal to that of a uniform rod by attached to the rod at both ends and suspended by the middle point
(A) the rod will sink until the total work done is non-zero.
(B) the rod will sink until the total work done is zero.
(C) sinking of rod is not determined or the basis of work done.
(D) sinking of rod is not possible.
62. A weight W is suspended from the midpoint of a rope, whose ends are at the same level. In order to make the rope perfectly horizontal, the force applied to each of its ends must be
(A) less than W
(B) equal to W
(C) equal to 2W
(D) infinitely large
63. The current gain of transistor is 100, if the base current changes by 10 μ What is the change in collector current?
(A) 0.2 mA
(B) 2 mA
(C) 1 mA
(D) 0.5 mA
64. Which of the following physical quantities has Neither dimensions Nor unit?
(A) Angle
(B) Luminous intensity
(C) Coefficient of friction
(D) Currents
65. Which of the following mode of propagation is used to send radio-waves from one place to another?
(A) Space wave propagation
(B) Sky wave propagation
(C) Ground wave propagation
(D) All of these
66. Six point charges are arranged at the vertices of a regular hexagon of side length a (shown in figure). The magnitude of electric field at the centre of regular hexagon is
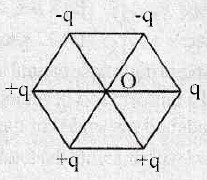
(A) 
(B) Zero
(C) 
(D) None of these
CHEMISTRY
(Questions-67-132)
67. The half-life period of a first order reaction is 10 minutes. The time required for the concentration of the reactant to change from 0.08 M to 0.02 M is
(A) 10 min.
(B) 20 min.
(C) 30 min.
(D) 40 min.
68. Which bond is the smallest?

69. The distance between two electrodes of a cell is 2.5 cm and area of each electrode is 5 cm2. The cell constant is
(A) 2
(B) 12.5
(C) 7.5
(D) 0.5
70. The conversion of PbO into Pb(NO3)2 involves
(A) oxidation
(B) reduction
(C) neither (A) nor (B)
(D) both (A) and (B)
71. A 0.6% urea solution would be isotonic with
(A) 0.1 M glucose solution
(B) 0.1 MKCI solution
(C) 0.6% glucose solution
(D) 0.6% NaCl solution
72. The direct change from solid to gaseous state is referred to as
(A) dissociation
(B) decomposition
(C) sublimation
(D) deliquescence
73. The maximum number of molecules present in
(A) 15 bL of H2 gas at STP
(B) 5L of N2 gas at STP
(C) 0.5 g of H2 gas
(D) 10g of O2 gas
74. Which of the following statements is (are) WRONG?
(A) If the value of 1 = 0, the electron distribution is spherical.
(B) The shape of the orbital is given by magnetic quantum no.
(C) Angular moment of 1s, 2s, 3s electrons are equal
(D) In an atom, all electrons travel with the same velocity.
75. Chloroform
(A) if exposed to air and light forms poisonous compound.
(B) if inhaled for long time effects central nervous system.
(C) is used to prepare chlorofluoromethane a Freon refrigerant.
(D) All of these
76. Aspirin is a(n)
(A) narcotic
(B) antipyretic
(C) tranquillizer
(D) anaesthetic
77. Which of the following is (are) NOT true?
(A) The most radioactive element present in pitchblende is uranium.
(B) 32P is used for the treatment of leukaemia.
(C) CO2 present in the air contains 12C only.
(D) None of these
78. On which factors interface depends?
(A) Size of the molecules in the bulk phase.
(B) Weight of the molecules in the bulk phase.
(C) Numbers of molecules in the bulk phase.
(D) Physical state of molecules in the bulk phase.
79. According to kinetic theory of gases
(A) the pressure exerted by gas is proportional to mean square velocity of the molecules.
(B) the pressure exerted by the gas is proportional t the root mean square velocity of the molecules.
(C) the mean translational KE of the molecule is directly proportional to the absolute temperature
(D) Both (B) and (C).
80. When copper ore is mixed with silica, in a reverberatory furnace copper matte is produced. The copper matte contains
(A) sulphides of copper (II) and iron (II)
(B) sulphides of copper (II) and iron (III)
(C) sulphides of copper (I) and iron (II)
(D) sulphides of copper (I) and iron (III)
81. Which plot represents an exothermic reaction?

82. Which statement about aspirin is NOR true?
(A) Aspirin belongs to narcotic analgesics.
(B) It is effective in relieving pain.
(C) It has antiblood clotting action.
(D) It is a neurologically active drug.
83. In a first order reaction A→B, if k is rate constant and initial concentration of the reactant A is 0.5 M then half-life is
(A) ![]()
(B) ![]()
(C) ![]()
(D) ![]()
84. Which of the following will NOT affect the value of equilibrium constant of a reaction?
(A) Change in the concentration of the reactants
(B) Change in temperature
(C) Addition of catalyst
(D) All of these
85. Glucose reacts with excess of phenylhydrazine and forms
(A) sorbitol
(B) glucose phenylbyrazone
(C) glucosaszone
(D) glucose oxime
86. The entropy of a crystalline substance at absolute zero on t he basis of third law of thermodynamics should be taken as
(A) 100
(B) 50
(C) zero
(D) different of different substances
87. Which of the following would undergo Hofniann’s reaction to give a primary amine?
(A) RCONH2
(B) RCONHCH3
(C) RCOCI
(D) RCOOR
88. Propene reacts with carbon monoxide and hydrogen in presence of cobalt carbonyl catalyst at high temperature and pressure, to form
(A) propanal
(B) butanal
(C) butanone
(D) butanoic acid
89. The hybrid state of central oxygen atom in diethylether is
(A) sp2
(B) sp3
(C) sp
(D) sp3d
90. The general molecular formula, which represents the homologous series of alkanol is
(A) CnH2nO
(B) CnH2n – 1O
(C) CnH2n – 2O
(D) CnH2nO2
91. The phenomenon of optical activity will be shown by

92. The compound used in enrichment of uranium for nuclear power plant is
(A) U3O8
(B) UF6
(C) UO2(NO3)2
(D) UCl4
93. The sweetest of all sugars is
(A) glucose
(B) lactose
(C) sucrose
(D) fructose
94. In which case, the carbon-carbon bond length is same?
(A) 2-butene
(B) benzene
(C) I-butene
(D) I-propyne
95. Which is a correct relationship?
(A) 
(B) ![]()
(C) ![]()
(D) All of these
96. A crystal lattice with alternative +ve and –ve ions has radius ratio 0.52, its coordination number is
(A) 4
(B) 3
(C) 6
(D) 12
97. ______bond is the most polar
(A) C – O
(B) C – Br
(C) C – S
(D) C – F
98. Blood cells do NOT shrink in blood because blood is
(A) hypertonic
(B) isotonic
(C) equimolar
(D) hypotonic
99. Which of the following is known as Freon which is used as a refrigerant?
(A) CCl2F
(B) CHCl3
(C) CH2F2
(D) CF4
100. The credit for the discovery of transuranic elements goes to
(A) Hahn
(B) Rutherford
(C) Seaborg
(D) Curie
101. What is used to prevante electronic instruments clamaged by the moisture?
(A) Silica gel
(B) Zeolite
(C) Chromatographic plate
(D) All of these
102. dz2 orbital has
(A) a lobe along z-axis and a ring along xy-plane
(B) a lobe along z-axis and a along xy-place.
(C) a lobe along z-axis and a ring along yz-plane
(D) a lobe and ring along z-axis
103. In the extraction of copper from its sulphide ore, the metal is formed by the reduction of Cu2O with
(A) FeS
(B) CO
(C) Cu2S
(D) SO2
104. Select the WRONG statement.
(A) One curie = 3.7 × 1010 dis/minute
(B) Actinium series starts with U238
(C) Both (A) and (B)
(D) None of these
105. Compound which is added to soap to impart antiseptic properties is
(A) sodium laurylsulphate
(B) sodium dodecylebenzenesulphonate
(C) rosin
(D) bithional
106. In the following statements,
(a) Ideal gases are liquefied only at very low temperatures
(b) Ideal gases cannot be liquefied.
(c) Ideal gas behavior is observed by real gases at low pressures.
(d) Ideal gases do not exist.
the correct statements are
(A) a, b, c and d
(B) a, b and c
(C) b, c and D
(D) c and d
107. Decron is an example of
(A) polyamide
(B) polypropylene
(C) polyurethane
(D) polyester
108. In the evaporation of water, the entropy
(A) decreases
(B) increases
(C) does not change
(D) sometimes increases, sometimes decreases
109. A compound is formed by elements A and B. This crystallizes in the cubic structure when atoms A are at the corners of the cube and atoms B are at the centre of the body. The simplest formula of the compound is
(A) AB
(B) AB2
(C) A2B
(D) AB4
110. Dead burnt plaster is
(A) CaSO4
(B) ![]()
(C) CaSO4H2O
(D) CaSO42H2O
111. Semipenneable membrane is that which pennits the passage of
(A) Solute molecules only
(B) Solvent molecules only
(C) Both (A) and (B)
(D) Neither (A) nor (B)
112. Which is non-aromatic compound?
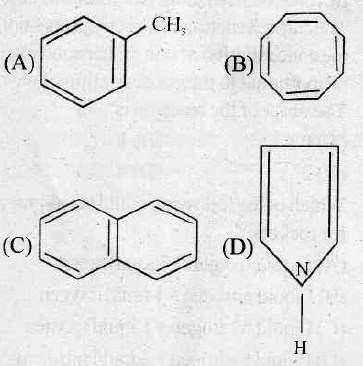
113. When one coulomb’ of electricity is passed through an electrolytic solution, the mass deposited on the electrode is equal to
(A) equivalent weight
(B) molecular weight
(C) electrochemical equivalent
(D) one gram
114. Among the following compounds, which one is NOT responsible for depletion of ozone layer?
(A) CH4
(B) CFCl3
(C) NO
(D) Cl2
115. The plot between concentration versus time for a zero order reaction is represented by
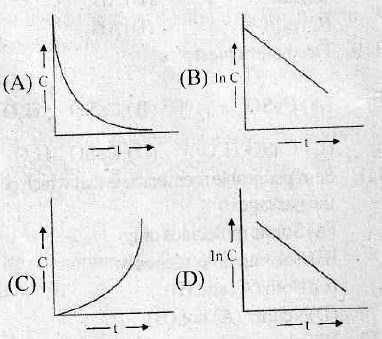
116. In a chemical reaction, two reactants take part. The rate of reaction is directly proportional to the concentration of one of them and inversely proportional to the concentration of the other. The order of the reaction is
(A) 0
(B) 1
(C) 2
(D) 4
117. Which of the following could act as propellant for rockets?
(A) Liquid oxygen + Liquid argon
(B) Liquid nitrogen + Liquid oxygen
(C) Liquid hydrogen + Liquid oxygen
(D) Liquid hydrogen + Liquid nitrogen
118. Heating mixture of Cu2O and Cu2S will give
(A) Cu + SO2
(B) Cu + SO3
(C) CuO + CuS
(D) CU2SO3
119. p-nitrophenol and o-nitrophenol are separated by
(A) cystallisation
(B) fractional distillation
(C) distillation
(D) steam distillation
120. Which of the following has magnesium ?
(A) Carbonic anhydrase
(B) Vitamin B12
(C) Chlorophyll
(D) Haemocyanine
121. Primary amine + aldehyde → X, what is X?
(A) Nitro
(B) Nitroso
(C) Amino
(D) Imino
122. Which graph will show equilibrium condition?

123. Propyne on hydroboration-oxidation gives mainly
(A) propanone
(B) propanoic acid
(C) propanal
(D) propane
124. The oxidation numbers of C in CH4, CH3HI CH2Cl, CHCI3 and CCl4 are respectively
(A) +4, +2, 0, −2, −
(B) +2, +4, 0, −4, −2
(C) −4, −2, 0, +2, +4
(D) −2, −4, 0, +4, +2
125. The reagent used for the preparation of higher ethers from halogenated ethers is
(A) conc. H2SO4
(B) sodium alkoxide
(C) dry silveroxide
(D) Grignard reagent
126. Heavy water is used in atomic reactors as
(A) coolant
(B) moderator
(C) Both (A) and (B)
(D) Neither (A) Nor (B)
127. CH3OH and C2H5OH may be distinguished chemically
(A) by the action of HCl
(B) by the action of I2 + NaCO3
(C) by the action of NH3
(D) solubility in water
128. Flurosis, a bone disease, is caused by the presence of
(A) pesticides in water
(B) fluorides in water
(C) carbon monoxide in air
(D) sulphur dioxide in air
129. Which one of the following cyano complexes would exhibit the lowest value of paramagnetic behavior?
(A) [Cr(CN)6]3−
(B) [Co(CN)6] 3−
(C) [Fe(CN)6] 3−
(D) BaCO3
130. Metal carbonates decompose on heating to give metal oxide and carbon dioxide. Which of the metal carbonates is most stable thermally?
(A) MgCO3
(B) CaCO3
(C) SrCO3
(D) BaCO3
131. Which of the following is NOT an actinide ?
(A) Curium
(B) Califomium
(C) Uranium
(D) Terbium
132. The major product formed by monobromination of methy chyclopentane is

MATHEMATICS
Questions – 133-200
133. Four natural numbers are selected at random and are multiplied. The probability that the product is divisible by 5 or 10 is
(A) 49/625
(B) 369/625
(C) 64/625
(D) 256/625
134. Let  satisfied A2 + aA + bI = 0, then a, b are respectively equal to
satisfied A2 + aA + bI = 0, then a, b are respectively equal to
(A) −4, 2
(B) −3, 3
(C) −4, 1
(D) −3, 1
135. The mean of 5 observations is 4.4 and the variance is 8.24. If three of the five observations are 1, 2 and 6, the two values are
(A) 4 and 9
(B) 3 and 5
(C) 2 and 6
(D) 4 and 6
136. Three numbers are chosen at random without replacement from {1, 2, 3 ……., 10}. The probability that minimum of the chosen number is 3 or their maximum is 7, is
(A) 11/30
(B) 11/40
(C) 1/7
(D) 1/8
137. The equations of perpendicular bisectors of the sides AB and AC of a triangle ABC are x – y + 5 = 0 and equation of the line BC is
(A) 23x + 14y – 40 = 0
(B) 23x + 14y + 40 = 0
(C) 14x + 23y – 40 = 0
(D) 14x + 23y + 40 = 0
138. If  then
then ![]() is
is
(A) 2
(B) 1
(C) 0
(D) x
139. Consider the proposition : “If we control population growth, we prosper”. Negative of this proposition is
(A) If we do not control population growth, we prosper.
(B) If we control population, we do not prosper.
(C) We control population but we do not prosper.
(D) We do not control population but we prosper.
140. If the tangent at (1, 1) on y2 = x(2 – x)2 meets the curve gain at P, then P is
(A) (4, 4)
(B) (−1, 2)
(C) (9/4, 3/8)
(D) None of these
141. The value of the sum of the series 3nC0 – 8nC1 + 13nC2 – 18nC3 + …. upon (n + 1) terms, is
(A) 0
(B) 3n
(C) 5n
(D) None of these
142. The value of  is
is
(A) π
(B) π/6
(C) π/3
(D) π/4
143. If the letters of the word MOTHER are written in all possible orders and these words are written out as in a dictionary, then the rank of the word MOTHER is
(A) 240
(B) 261
(C) 308
(D) 309
144. An equation of the curve in which subnormal varies as the square of the ordinate is (k is constant of proportionality)
(A) y = Aekx
(B) y = ekx
(C) 
(D) y2 + kx2 = A
145. The sum of the series (1 + 2) + (1 + 2 + 22) + (1 + 2 + 22 + 23)+ …. upto n terms is
(A) 2n+2 – n – 4
(B) 2(2n – 1)
(C) 2n+1 – n
(D) 2n+1 – 1
146. Let there be two points A, B on the curve y = x2 in the plane OXY satisafying OA I = 1 and OB I = −2 then the length of the vector 2OA – 3OB is
(A) √14
(B) 2√51
(C) 3√41
(D) None of these
147. If n is a positive integer greater than unity and z is a complex number satisfying the equation zn = (z + 1)n, then
(A) Im (z) < 0
(B) Im (z) > 0
(C) Im (z) = 0
(D) None of these
148. Let [x] denotes the greatest integer less than or equal to x. If f(x) = sin−1x, g(x) [x2] and ![]()
(A) fogohh(x) = π/2
(B) fogpoh(x) = π
(C) hofog = hogof
(D) hofog ≠ hogof
149. Let f : N → N be defined by f(x) = x2 + x + 1, then f is
(A) one-one onto
(B) many one onto
(C) one-one but not into
(D) None of these
150. 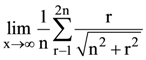 equals
equals
(A) 1 + √5
(B) −1 + √5
(C) −1 + √2
(D) 1 + √2
151. Let R be a relation on the set N be defined by {(x, y) |x, y ∈ N, 2x + y = 41}. Then R is
(A) reflexive
(B) symmetric
(C) transitive
(D) None of these
152. Let = {1, 2, 3}, B = {3 4}, C = {4, 5, 6}. Then A ⋃ (B⋂C) is
(A) {3}
(B) {1, 2, 3, 4}
(C) {1, 2, 5, 6)
(D) {1, 2, 3, 4, 5, 6}
153. If  decreases for all x, then
decreases for all x, then
(A) ab – bc < 0
(B) ad – bc < 0
(C) ab – cd > 0
(D) ab – cd < 0
154. If  then at x = 0
then at x = 0
(A) f(x) has no limit
(B) f(x) is discontinuous
(C) f(x) is continuous but not differentiable
(D) f(x) is differentiable
155. 
(A) √2
(B) Does not exist
(C) 1
(D) −√2
156. An unbiased cubical die is thrown 5 times. The probability that the maximum number appearing on the die is 4 is
(A) 7/65
(B) 1023/65
(C) 3871/65
(D) 1781/65
157. The distance between the lines 5x – 12y + 65 = 0 and 5x – 12y – 39 = 0, is
(A) 4
(B) 16
(C) 2
(D) 8
158. If ω ≠ 1 is a cube root of unity and 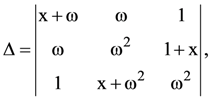 then value of x is
then value of x is
(A) 0
(B) 1
(C) −1
(D) None of these
159. If p and q are two propositions, then ~ (p ↔ q) is
(A) ~ p ^ ~ q
(B) ~p ⋁ (~ p ^ q)
(C) Both (A) and (B)
(D) None of these
160. The distance between the line r = 2is – 2j + 3k + λ (i – j + 4k) and the plane r.(i + 5j + k) = 5 is
(A) 3/10
(B) 10/3
(C) 10/9
(D) 10/3√3
161. Common roots of the equation z3 + 2z2 + 2z + 1 = 0 and z1985 + z100 + 1 = 0 are bijection?
(A) w, w2
(B) 1, w, w2
(C) −1, w, w2
(D) −w, w2
162. Which of the following functions from Z to itself are bijection?
(A) f(x) = x3
(B) f(x) = x + 2
(C) f(x) = 2x + 1
(D) f(x) = x2 + x
163. Let 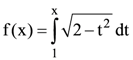 Then the real roots of the equation x2 – f′(x) = 0 are
Then the real roots of the equation x2 – f′(x) = 0 are
(A) ±1
(B) ±1/√2
(C) ±1/2
(D) 0 and 1
164. Let A = (p, q, r) Which of the following is NOT an equivalence relation on A?
(A) R1 = {(p, q), (q, r), (p, r), (p, p)}
(B) R2 = {(r, q), (r,p), (r, r), (p, q)}
(C) R3 = {(p, p), (q, q), (r, r), (p, q)}
(D) None of these
165. A particular solution of ![]() is
is
(A) e3x + 3e−4y
(B) 4e3x – e−4y = 3
(C) 3e3x + 4e4y = 7
(D) 4e3x + 3e−4y = 7
166. The area of the region bounded by the curves y = x2 + 2, y = x, x = 0 and x= 3, in square units is
(A) 21/4
(B) 21/2
(C) 39/2
(D) 39/4
167. If sets A and B are defined as
A = {(x, y) : y = ex, x ∈ R}
B = {(x, y) : y = x, x ∈ R}, then
(A) B ⊂ A
(B) A ⊂ B
(C) A ⋂ B = ϕ
(D) A ⋃ B = A
168. If the line ![]() lies in the plane x + 3y – az + β = 0. Then (α, β) = ?
lies in the plane x + 3y – az + β = 0. Then (α, β) = ?
(A) (5, −15)
(B) (−5, 5)
(C) (6, −17)
(D) (−6, 7)
169. If R is a relation from a set A to a set B and S is relation from a set B to a set C, then the relation SoR
(A) is from A to C
(B) is from C to A
(C) does not exist
(D) None of these
170. The non-zero vectors a, b and c are related by a = 8b and c = −7b. Then the angle between a and c is
(A) 0
(B) π/4
(C) π/2
(D) π
171. Let F : A → B and g : B → A be two functions such that gof = IA. Then
(A) f is an injection and g is a surjection
(B) f is a surjection and g is an injection
(C) f and g both are injections
(D) f and g both are surjections
172. The solution of the equation (2x + y + 1)dx + (4x + 2y – 1) dy =0 is
(A) log |2x + y – 1| = C + x + y
(B) log (4x + 2y – 1) = C + 2x + y
(C) log(2x + y + 1) + x + 2y = C
(D) log|2x + y – 1| + x + 2y = C
173. If z1 = a + ib and z2 = c + id are complex numbers such that |z1| = |z2| = 1 and, then ![]() , then the pair of w2 = b + id satisfy
, then the pair of w2 = b + id satisfy
(A) |w1| = 1
(B) |w2| = 1
(C) ![]()
(D) All of these
174. The value of  is ([x] is the greatest integer function)
is ([x] is the greatest integer function)
(A) 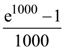
(B) 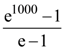
(C) 1000(e – 1)
(D) ![]()
175. If the interior angles of a polygon are in A.P. with common difference 5° and smallest angle is 120°, then the number of sides of the polygon is
(A) 9 or 16
(B) 9
(C) 16
(D) 13
176. If  then x is any term of the following.
then x is any term of the following.
(A) 3, 6, 9, 12, ……
(B) 9, 18, 27, 36, …
(C) 6, 12, 18, 24, ….
(D) 6/5, 12/5, 18/5, ….
177. Let f(x) = sgn(sgn(sgn x))
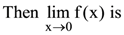
(A) 1
(B) 2
(C) 0
(D) None of these
178. If ![]() then 4x2 – 4xycosα + y 2 is equal to
then 4x2 – 4xycosα + y 2 is equal to
(A) 4 sin2 α
(B) −4 sin2 α
(C) −2 sin2 α
(D) 4
179. The statement ~ (p ↔ ~ q) is
(A) equilvalent to ~ p ↔ q
(B) a tautology
(C) a fallacy
(D) equivalent to p ↔ q
180. Suppose a, b, c, > 0 and a, b, c are the pth, qth, rth terms of a GP. Let
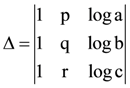
Then numerical value of ∆ is
(A) −1
(B) 2
(C) 0
(D) None of these
181. The total number of proper divisors or 38808 is
(A) 72
(B) 70
(C) 69
(D) 71
182. If letter of the word “ASSASSIN” are written down at random in a row, the probability that no two S’s occur together is
(A) 1/7
(B) 1/14
(C) 1/28
(D) 1/35
183. Let A, B, C be three square matrices of the same order, such that whenever AB = AC then B = C, if A is
(A) singular
(B) non-singular
(C) symmetric
(D) skew-symmetric
184. If 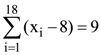 and
and  then the standard deviation of x1, x2, ….., x18 is
then the standard deviation of x1, x2, ….., x18 is
(A) 4/9
(B) 9/4
(C) 3/2
(D) None of these
185. The least number of times a fair coin must be tossed so that the probability of getting at least one head is 0.95 is
(A) 5
(B) 6
(C) 7
(D) 12
186. The equation of one side of a rectangle is 3x – 4y – 10 = 0 and the co-ordinates of two of its vertices are (−2, 1) and (2, 4). Then the area of the rectangle is
(A) 20 sq. units
(B) 40 sq. units
(C) 10 sq. units
(D) 30 sq. units
187. The slope of the tangent of the curve
x = t2 + 3t – 8
y = 2r2 – 2t – 5
at the point (2, −1) is
(A) 2/3
(B) 6/7
(C) 4/5
(D) 3/2
188. The least value of n so that yn = yn + 1 where y = x2 + ex is
(A) 4
(B) 3
(C) 5
(D) 2
189. The interval in which x must lie so that the numerically greatest term in the expansion of (1 – x)2 has the numerically greatest coefficient, if
(A) [5/6, 6/5]
(B) (5/6, 6/5)
(C) (4/5, 5/4)
(D) [4/5, 5/4)
190. 
(A) 1/2
(B) 3/2
(C) 5/2
(D) 7/2
191. Logical equivalent proposition to the proposition ~(p ⋁ q) is
(A) ~p ⋀ ~q
(B) ~p ⋁ ~q
(C) ~p → ~q
(D) ~p ↔ ~q
192. The nearest point on the line 3x – 4y = 25 from the origin is
(A) (−4, 5)
(B) (3, −4)
(C) (3, 4)
(D) (3, 5)
193. The distance of the point (1, −5, 9) from the plane x – y + z = 5 measured along a straight line x = y = z is
(A) 10√3
(B) 5√3
(C) 3√10
(D) 3√5
194. The unit vector which is orthogonal to the vector 5i + 2j + 6k and is coplanar with the vectors 2i + j + k and i – j + k is
(A) ![]()
(B) ![]()
(C) ![]()
(D) ![]()
195. If A is a finite set having n elements, then P(A) has _______ elements.
(A) 2n
(B) 2n
(C) n
(D) None of these
196. A function out of the following whose period is NOT π is
(A) sin2x
(B) cos2x
(C) tan(2x + 3)
(D) y = |sin x|
197. 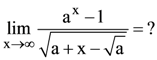
(A) 2√a log a
(B) √a log a
(C) log a
(D) None of these
198. If 0 ≤ x ≤ 1 and θ = sin−1 x + cos−1 x − tan−1 x, then
(A) ![]()
(B) ![]()
(C) ![]()
(D) ![]()
199. Which of the following is NOT a statement?
(A) 17 is a prime number.
(B) 22 is an odd number.
(C) What a beautiful flower !
(D) New Delhi is Capital of India
200. If a = ω ≠ 1 is cube root of unity, b = −785, c = 2008i, and 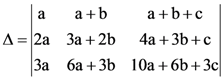
then ∆ equals
(A) −i
(B) i
(C) 1
(D) 1 – ωi
BIOLOGY
(Questions – 133-200)
133. Which is a part of pectoral girdle?
(A) Glenoid cavity
(B) Sternum
(C) Illium
(D) Acetabulum
134. Due to discovery of which of the following in 1980 the evolution was termed as RNS world?
(A) mRNA, tRNA, TRNA synthesize proteins.
(B) In some virus RNA is genetic material.
(C) RNA have enzymatic property.
(D) RNA is not found in all cells.
135. Iris is part of
(A) sclerotic
(B) choroid/uvula
(C) choroid and retina
(D) sclerotic and choroid
136. Desert plants are generally
(A) viviparous
(B) succulent
(C) herbaceous
(D) heterophyllus
137. A condition of failure of kidney to form urine is called
(A) deamination
(B) entropy
(C) anuria
(D) None of these
138. In RNA, thymine is replaced by
(A) adenine
(B) guanine
(C) Cytosine
(D) Uracil
139. A transgenic food crop which may help in solving the problem of might blindness in developing countries is
(A) Flavr savr tomatoes
(B) Starling maize
(C) Bt soybean
(D) Golden rice
140. Escherichia coli is used extensively in biological research as it is
(A) easily cultured
(B) easily available
(C) easy to handle
(D) easily multiplied in host
141. Farmers have reported over 50% higher yields of rice By using which of the following biofertilizer?
(A) Mycorrhiza
(B) Azollapinnata
(C) Cyanobacteria
(D) Legume-Rhizobium symbiosis
142. The part of life cycle of malarial parasite plasmodium vivax, that is passed in female Anopheles is
(A) Sexual cycle
(B) Pre-erythrocytic schizogony
(C) Exo-erythrocytic schizogony
(D) Post-erythrocytic schizogony
143. The bacteria associated with plant genetic engineering are
(A) Salmonella and Pseudomonas
(B) Salmonella typhimurium and Agrobacterium
(C) Bacillus thuringiensis and Pseudomonas Fluorescens
(D) Both (B) and (C)
144. In plants inulin and pectin are
(A) reserved food material
(B) wastes
(C) secretory material
(D) insect attaching material
145. Middle piece of mammalian sperm possesses
(A) mitochondria and centriole
(B) mitochondria only
(C) centriole only
(D) nucleus and mitochondria
146. Photoreceptors of earthworm occur on
(A) clitellum
(B) many eyes
(C) dorsal surface
(D) lateral sides
147. Most diverse macromolecules, found in the cell both Physically and chemically are
(A) proteins
(B) carbohydrates
(C) nucleic acids
(D) lipids
148. The population of an insect species shows an explosive Increase in numbers during rainy season followed by its disappearance at the end of t he season. What does This show?
(A) S-shaped or sigmoid growth of this insect.
(B) The food plants mature and die at the end of the rainy season.
(C) Its population growth curve is of J-type
(D) The population of its predacors increases enormously.
149. Which is employed for artificial ripening of banna fruits?
(A) Auxin
(B) Cumarin
(C) Ethylene
(D) Cytokinin
150. Home sapiens evolved during
(A) Pleistocene
(B) Oligocene
(C) Pliocene
(D) Miocene
151. In which one of the following do the two names rafer to one and the same thing?
(A) Tricarboxylic acid cycle and Urea cycle
(B) Krebs cycle and Calvin cycle
(C) Tricarboxylic acid cycle and Citric acid cycle
(D) Cirric acid cycle and Calvin cycle
152. Which of the following is a reducing sugar?
(A) Galactose
(B) Gluconic acid
(C) B-methyl galactoside
(D) Sucrose
153. In plant cells, peroxisomes are associated with
(A) photorespiration
(B) phototropism
(C) photoperiodism
(D) photosynthesis
154. Which of the following plant species you would select for the production of bioethanol?
(A) Brassica
(B) Zea mays
(C) Pongamia
(D) Jatropha
155. Afferent nerve fibres carry impulses from
(A) effector organs to CNS
(B) receptors to CNS
(C) CNS to receptors
(D) CNS to muscles
156. Bio augmentation is
(A) the addition of commercially prepared bacterial Strain
(B) production of fertilizers by using bacteria
(C) the metals are deposited as insoluble oxides and sulphides by activities of bacteria
(D) removal of pests
157. Glycogen is a polymer of
(A) galactose
(B) glucose
(C) fructose
(D) sucrose
158. If the mean and the median pertaining to a certain Character of a population are of the same value, the following is most likely to occur.
(A) Normal distribution
(B) Bio-modal distribution
(C) T-shaped curve
(D) Skewed curve
159. Choose the correct match:
Bladderwort, Sundew, Venusfly trap
(A) Nepenthes, Diorea, Drosera
(B) Nepenthes, Utricularia, Vanda
(C) Utricularia, Drosera, Diorea
(D) Diorea, Trapa, Vanda
160. One of the following is the correct sequence to make a Transgenic animals.
(A) Transomics-Transfection Micro infection Electro portion – Retroviralvectors
(B) Micro injection Transfection Electro portion-
(C) Retroviral vectors Transomics Transfection – Micro injection – Transomics Electro portion – Retroviral vectors
(D) None of these
161. Protein synthesis in an animal cell takes place
(A) only in the cytoplasm
(B) in the nucleolus as well as in cytoplasm
(C) in cytoplasm as well as it mitochondria
(D) Only on ribosomes attached to the nuclear Envelope
162. Flowering dependent on cold treatment in
(A) cryotherapy
(B) cryogenics
(C) cryoscopy
(D) vernalization
163. Transgenic plants are produced by using Ti Plasmids From the
(A) Agrobacterium tumefaciens
(B) E. coli
(C) Bacteriophage
(D) Agrobacterium varians
164. The plant group that produces spores and embryo but Lacks vascular tissues and seeds is
(A) Pteridophyta
(B) Rhodophyta
(C) Bryophyta
(D) Phaeophyta
165. Removal of apical bud results in
(A) formation of new apical buid
(B) elongation of main stem
(C) death of plant
(D) Formation of lateral branching
166. In pinus, the pollen grain has 6 chromosomes then its Endosperm will have the chromosome
(A) 12
(B) 18 n 8
(C) 6
(D) 24
167. The foods made from genetically modified crops required to Pass human testing because
(A) they may cause allergies
(B) they may alter genes
(C) they may cause mutations and release toxins
(D) All of these
168. Aquatic reptiles are
(A) ammonotelic
(B) ureotelic
(C) ureotelic in water
(D) ureotelic over land
169. Function of iris is to
(A) move lens forward and backward
(B) refract light rays
(C) bring about movements of eye lids
(D) alter the size of pupil
170. The aquatic fem, which is an excellent biofertilizer, is
(A) Azolla
(B) pteridium
(C) Salvinia
(D) Marselia
171. In a standard ECG, which one of the following Alphabets is the correct representation of the respective Activity of the human heart?
(A) R-repolarisation of ventricles
(B) S-start of systole
(C) T-end of diastole
(D) P-depolarisation of the atria
172. Total number of bones in the hind limb of man is
(A) 14
(B) 30
(C) 24
(D) 21
173. The most environmental hazards were created by Accidents in nuclear power plant and MIC gas tragedy Respectively in
(A) Russia in 1990 and Bhopal in 1986
(B) Ukrain in 1988 and USA in 1984
(C) Bhopal in 1984 and Russia in 1990
(D) Ukrain in 1986 and Bhopal 1984
174. Meiosis-II performs
(A) separation of sex chromosomes
(B) synthesis of DNA and centromeres
(C) separation of homologous chromosomes
(D) separation of chromatids
175. Sequence of which of the following is used to know the Phylogeny?
(A) mRNA
(B) rRNA
(C) tRNA
(D) DNA
176. During which stage, in the complete oxidation of Glucose are the greatest number of ATP molecules Formed from ADP?
(A) glycolysis
(B) krebs cycle
(C) conversion of pyruvic acid to acetyl Co-A
(D) electron transport chain
177. Darwin’s finches provide an excellent evidence in Favour of evolution. This evidence comes from the field of
(A) Biogeography
(B) Anatomy
(C) Embryology
(D) Palaeontology
178. Movement of auxins is
(A) Centripetal
(B) basipetal
(C) Acropetal
(D) Both (B) and (C)
179. Agene pair hides the effect of another. The phenomenon is
(A) epistasis
(B) dominance
(C) mutation
(D) None of these
180. Radioactive thymidine when added to the medium surrounding living mammalian cells gets incorporated into the newly synthesized DNA. Which of the following types of chromatin is expected to become Radioactive if cells are exposed radioactive radioactive thymidine As soon as they enter the S-shape?
(A) Heterochromatin
(B) Euchromatin
(C) Both (A) and (B)
(D) Neither heterochromatin nor euchromatin but only the nucleolus
181. Which one of the following is the correct statement regarding the particular psychotropic drug specified?
(A) Hashish causes alter thought perceptions and Hallueinations.
(B) Opium stimulates nervous system and causes Hallucinations.
(C) Morphine leads to delusions and distributed Comolions.
(D) Barbiturates cause relaxation and temporary Euphoria.
182. Flight muscles of bird are attached to
(A) clavicle
(B) keel of sternum
(C) scapul
(D) coracoid
183. During cleavage, what is TRUE about cells?
(A) Nucleocytoplasmic ratio remains unchaged.
(B) Size does not increase.
(C) There is less consumption of oxygen.
(D) The division is like meiosis.
184. Pyrenoids are the centres for formation of
(A) prophyra
(B) enzymes
(C) fat
(D) starch
185. Who discovered plasmodium in RBC of human beings?
(A) Ronald Ross
(B) Mendel
(C) Laveran
(D) Stephen
186. Modifications by germ line gene therapy are heritable as the Functional gene is incorporated into
(A) their genome
(B) one of the gene
(C) Somatic cells
(D) All of these
187. In soil, water available for roots (to plants) is
(A) capillary water
(B) hygroscopic water
(C) gravitational water
(D) chemically bound water
188. Suppression of reproduction of one type of organism of utilizing some features of its biology or Physiology to destroy it or by use of another organism is known as
(A) Competition
(B) Predation
(C) Biological control
(D) Physiological control
189. Which one of the following proved effective for biological control of nematodal diseases plants?
(A) Pisoighusrinctorius
(B) Pseudomonas cepacia
(C) Glioclodiumvircns
(D) Paccilomyceslilacinus
190. Which one belongs to Monera?
(A) Amoeba
(B) Escherichia
(C) Gelidium
(D) Spirogyra
191. The Taj Mahal is threatened due to the effect of
(A) Oxygen
(B) Hydrogen
(C) Chlorine
(D) Sulphur dioxide
192. Study of fossils is
(A) Palaeoncology
(B) Herpetology
(C) Saurology
(D) Organic evolution
193. A patient suffering from cholera is given saline drip because
(A) Cl-ions are important component of blood plasma
(B) Nations help to retain water in the body
(C) Na+ ions are important in transport of substances across membrane
(D) Cl-ions help in the formation of HCl in stomach For digestion
194. Gonads develop from embryonic
(A) ecodermn
(B) endoderm
(C) mesoderm
(D) Both (B) and (C)
195. Which of the following cranial nerves can regulate Heart beat?
(A) X
(B) IX
(C) VIII
(D) VII
196. Random genetic drift in a population probably results from
(A) constant low mutation rate
(B) large population size
(C) highly genetically variable individuals
(D) interbreeding within this population
197. A deltoid ridge occurs in
(A) radius
(B) ulna
(C) femur
(D) humerus
198. The contrasting pairs of factors in Mendelian crosses are called
(A) multiple alleles
(B) allelomorphs
(C) aliolori
(D) paramorphs
199. Golgi apparacus is absent in
(A) higher plans
(B) Yeast
(C) bacteria and orange-green algae
(D) None of these
200. In general, in the developmental history of a of Mammalian heart, it is observed that it passes through A two-chambered fish like heart, three-chambered a Frog like heart and finally to four-chambered stage. To which hypothesis can this above cited statements by approximated?
(A) Hardy-Weinberg law
(B) Lamarkc’s principle
(C) Biogenetic law
(D) Mendelian principles
Latest Govt Job & Exam Updates: